Anupriya Adhikari
Department of Chemistry, Graphic Era Hill University, Dehradun, Uttarakhand, India
K Ganesh Kadiyala
Department of Chemistry, Shri Vishnu Engineering College for Women, Bhimavaram, Andhra Pradesh, India
Mamta Bisht
Department of Chemistry, School of Applied and Life Sciences, Uttaranchal University, Dehradun, Uttarakhand, India
Neetu Sharma
Department of Chemistry, Graphic Era (Deemed to be) University, Dehradun, Uttarakhand, India
Bhawana Bisht
Department of Chemistry, Graphic Era (Deemed to be) University, Dehradun, Uttarakhand, India
Keywords: Zn(II) complex DNA artificial nuclease cancer
Abstract
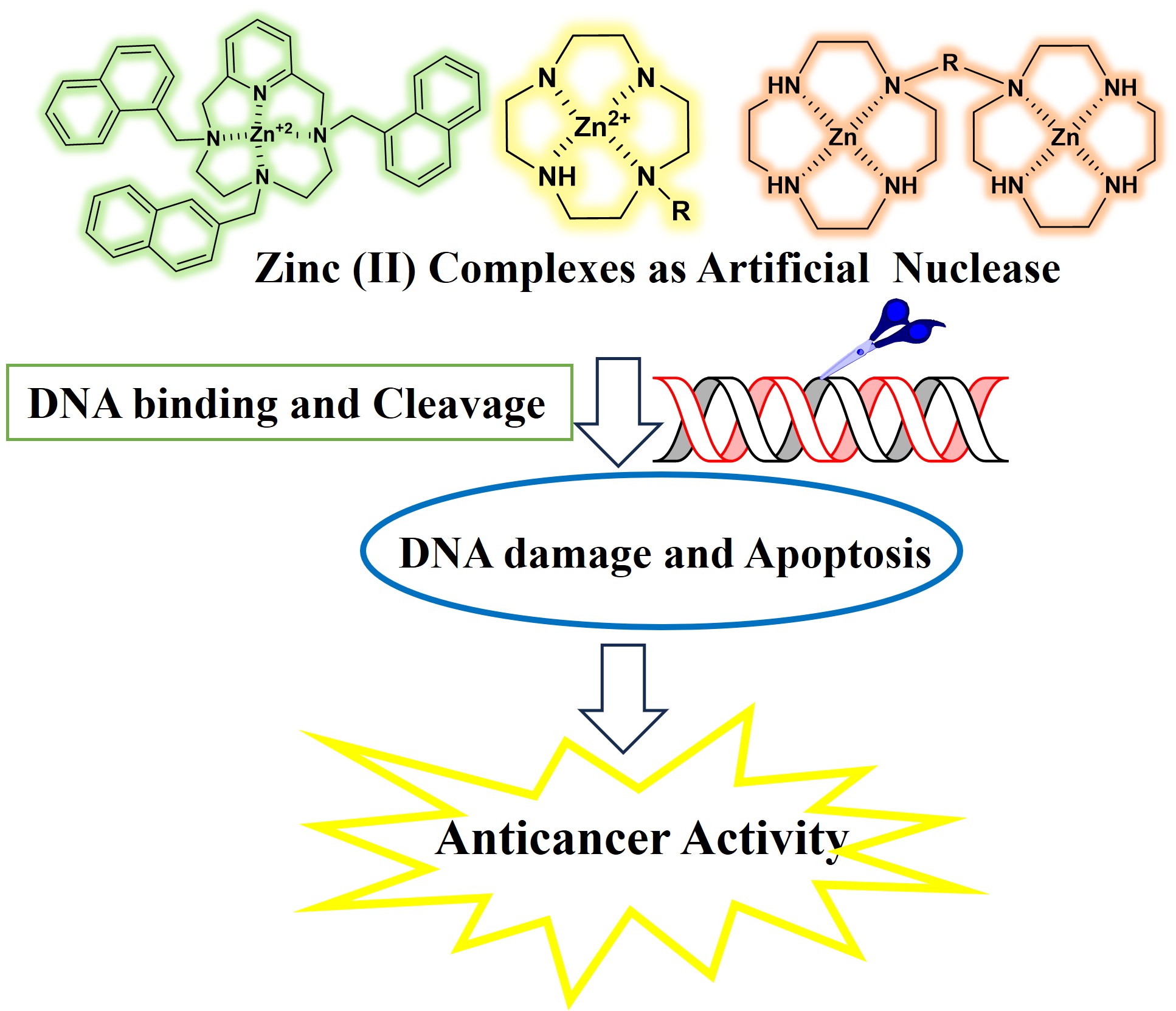
Zinc (II) complexes have recently gained attention for their unique ability to mimic natural nucleases and selectively target deoxyribonucleic acid (DNA), making them attractive candidates in the search for more effective and less toxic anticancer agents. This review explores the evolving landscape of Zn (II)-based artificial nucleases, focusing on how structural modifications—such as macrocyclic scaffolds, aromatic appendages, and bio-relevant ligands—enhance their ability to bind and cleave DNA. These complexes operate through both hydrolytic and oxidative pathways, disrupting genetic material and triggering programmed cell death in cancer cells. Their redox stability, biocompatibility, and catalytic efficiency offer distinct advantages over other metal-based systems. By bridging the fields of inorganic chemistry and oncology, these zinc complexes show great potential not only as therapeutic agents but also as molecular tools in gene editing and biomedical research. This review brings together recent findings to provide a clearer understanding of how Zn (II) based systems function and where their future applications might lie.
How to Cite
Adhikari, A., Kadiyala, K. G. ., Bisht, M., Sharma, N. ., & Bisht, B. (2025). Zinc (II) metal appended Artificial Nucleases as Anticancer Agents: A Brief Review. Asia-Pacific Journal of Science and Technology, 30(06), APST–30. https://doi.org/10.14456/apst.2025.87
References
Min HY, Lee HY. Molecular targeted therapy for anticancer treatment. Exp Mol Med. 2022;54(10):1670–1694.
Srinivasan A, Gold B. Small-molecule inhibitors of DNA damage-repair pathways: An approach to overcome tumor resistance to alkylating anticancer drugs. Future Med Chem. 2012;4(9):1093–1111.
Emrani J. Changing nature of diagnosis and treatment of cancer. Biomed Res Net. 2018;10(4):1-4.
Palchaudhuri R, Hergenrother PJ. DNA as a target for anticancer compounds: methods to determine the mode of binding and the mechanism of action. Curr Opin Biotechnol. 2007;18(6):497–503.
Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002; 2(3):188–200.
McKie SJ, Neuman KC, Maxwell A. DNA topoisomerases: Advances in understanding of cellular roles and multi-protein complexes via structure-function analysis. Bioessays. 2021;43(4):e2000286.
Jedrzejas MJ, Setlow P. Comparison of the binuclear metalloenzymes diphosphoglycerate-independent phosphoglycerate mutase and alkaline phosphatase: Their mechanism of catalysis via a phosphoserine intermediate. Chem Rev. 2001;101(3):607–618.
Cowan JA. Metal activation of enzymes in nucleic acid biochemistry. Chem Rev. 1998;98(3):1067–1088.
Gates KS. An overview of chemical processes that damage cellular DNA: Spontaneous hydrolysis, alkylation, and reactions with radicals. Chem Res Toxicol. 2009;22(11):1747–1760.
Loginova NV, Harbatsevich, HI, Osipovich, NP, Ksendzova, GA, Koval’chuk, TV, & Polozov, GI. Metal complexes as promising agents for biomedical applications. Curr Med Chem. 2020;27(31):5213–5249.
Haas KL & Franz, KJ. Application of metal coordination chemistry to explore and manipulate cell biology. Chem Rev. 2009;109(10):4921–4960.
Liu C, Wang M, Zhang T, Sun H. DNA hydrolysis promoted by di- and multi-nuclear metal complexes, Coord. Chem. Rev. 2004;248 (1–2):147-168.
Arjmand F, Parveen S, Mohapatra DK. Synthesis, characterization of Cu (II) and Zn (II) complexes of proline-glycine and proline-leucine tetrapeptides: In vitro DNA binding and cleavage studies, Inorg. Chim. Acta, 2012;388:1–10.
Jiang Q, Xiao N, Shi P, Zhu Y and Guo Z. Design of artificial metallonucleases with oxidative mechanism. Coord Chem Rev. 2007;25:1951–1972.
Hiraku Y, Ito K, Hirakawa K, Kawanishi S. Photosensitized DNA damage and its protection via a novel mechanism. Photochem Photobiol. 2007;83(1):205–212.
Shionoya M, Kimura E. and Shiro M. A new ternary Zinc (II) complex with [12] aneN4 (=1,4,7,10-tetraazacyclododecane) and AZT (=3′-azido-3′-deoxythymidine). Highly selective recognition of thymidine and its related nucleosides by a Zinc (II) macrocyclic tetraamine complex with novel complementary associations. J Am Chem Soc. 1993;115:6730–6737.
Kikuta E, Murata M, Katsube N, Koike T. and Kimura E. Novel recognition of thymine base in double-stranded dna by Zinc (II)−macrocyclic tetraamine complexes appended with aromatic groups. J Am Chem Soc. 1999;121:5426–5436.
Akhtar MJ, Ahamed M, Kumar S, Khan MM, Ahmad J, & Alrokayan SA. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomed. 2012;7:845–857.
Linder DP, Baker BE, Rodgers KR, [(H2O) Zn (Imidazole)n] 2+: the vital roles of coordination number and geometry in Zn–OH2 acidity and catalytic hydrolysis. Phys Chem Chem Phys. 2018;20:24979–24991.
Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium (III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem Rev. 1999;99(9):2293–2352.
Leivers M, Breslow R. Concerning two-metal cooperativity in model phosphate hydrolysis. Bioorg Chem. 2001;29(6):345–356.
König B, Pelka M, Subat M, Dix I, Jones PG. Urea derivatives of 1,4,7,10-tetraazacyclododecane − synthesis and binding properties. Eur J Org Chem. 2001;2001(10):1943–1949.
Subat M, Woinaroschy K, Anthofer S, Malterer B, König B. 1,4,7,10-tetraazacyclododecane metal complexes as potent promoters of carboxyester hydrolysis under physiological conditions. Inorg Chem. 2007;46(10):4336-4356.
E. Kimura, Y. Kodama, T. Koike, M. Shiro. Phosphodiester hydrolysis by a new Zinc (II) macrocyclic tetraamine complex with an alcohol pendant: Elucidation of the roles of ser-102 and Zinc (II) in alkaline phosphatase. J Am Chem Soc. 1995;117(32):8304–8311.
Kalesse M, Loos A. Transesterification of phosphodiester by a zinc-containing cyclen derivative: identification of the active species. Liebigs Ann. 1996;1996(6):935–939.
Pellei M, Bello FD, Porchia M, Santini C. Zinc coordination complexes as anticancer agents, Coord. Chem Rev. 2021;445:214088.
Li QL, Huang J, Wang Q, et al. Monometallic complexes of 1,4,7,10-tetraazacyclododecane containing an imidazolium side: synthesis, characterization, and their interaction with plasmid DNA. Bioorg Med Chem. 2006;14(12):4151–4157.
Wan SH, Liang F, Xiong XQ, et al. DNA hydrolysis promoted by 1,7-dimethyl-1,4,7,10-tetraazacyclododecane. Bioorg Med Chem Lett. 2006;16(10):2804–2806.
Tucker JHR, Shionoya M, Koike T. and Kimura E. A Zinc (II)–cyclen complex attached to an anthraquinone moiety that acts as a redox-active nucleobase receptor in aqueous solution. Bull Chem Soc Jpn. 1995;68:2465–2469.
Aït-Haddou H, Sumaoka J, Wiskur SL, Folmer-Andersen JF, Anslyn EV. Remarkable cooperativity between a Zn II ion and guanidinium/ammonium groups in the hydrolysis of RNA. Angew Chem. 2002;41(21):4013–4016.
Xia CQ, Jiang N, Zhang J, et al. The conjugates of uracil-cyclen Zn (II) complexes: synthesis, characterization, and their interaction with plasmid DNA. Bioorg Med Chem. 2006;14(16):5756-5764.
Shionoya M, Ikeda T, Kimura E. and Shiro M. Novel “multipoint” molecular recognition of nucleobases by a new Zinc (II) complex of acridine-pendant cyclen (cyclen = 1,4,7,10-tetraazacyclododecane). J Am Chem Soc. 1994;116:3848–3859.
Anbu S, Kamalraj S, Varghese B, Muthumary J, & Kandaswamy M. A series of oxyimine-based macrocyclic dinuclear zinc (II) complexes enhances phosphate ester hydrolysis, DNA binding, DNA hydrolysis, and lactate dehydrogenase inhibition and induces apoptosis. Inorg Chem. 2012:51(10): 5580–5592.
Wen JH, Li CY, Geng ZR, Ma XY, & Wang ZL. A potent antitumor Zn2+ tetraazamacrocycle complex targeting DNA: the fluorescent recognition, interaction and apoptosis studies. Chem Comm. 2011;47(40):11330–11332.
Montagner D, Gandin V, Marzano C, Erxleben A. Phosphate Diester Cleavage, DNA Interaction and Cytotoxic Activity of a Bimetallic Bis(1,4,7-triazacyclononane) Zinc Complex. Eur J Inorg Chem. 2014;2014(25):4084–4092.
Aragoni MC, Arca M, Bencini A, et al. Coordination chemistry of N-aminopropyl pendant arm derivatives of mixed N/S-, and N/S/O-donor macrocycles, and construction of selective fluorimetric chemosensors for heavy metal ions. Dalton Trans. 2005;18:2994–3004.
Xiang QX, Zhang J, Liu PY, et al. Dinuclear macrocyclic polyamine zinc (II) complexes: syntheses, characterization and their interaction with plasmid DNA. J Inorg Biochem. 2005;99(8):1661–1669.
Dasmahapatra U., Maiti B., Alam MM, Chanda K. Anti-cancer property and DNA binding interaction of first row transition metal complexes: A decade update, Eur. J Med Chem. 2024;275:116603.
Adhikari A, Kumari N, Adhikari M, et al. Zinc complex of tryptophan appended 1,4,7,10-tetraazacyclododecane as potential anticancer agent: Synthesis and evaluation. Bioorg Med Chem. 2017;25(13):3483–3490.
Parveen S, Arjmand F, Mohapatra DK. Zinc (II) complexes of Pro-Gly and Pro-Leu dipeptides: synthesis, characterization, in vitro DNA binding and cleavage studies. J Photochem Photobiol B 2013;126:78–86.
Sheng X, Guo X, Lu XM, Lu GY, Shao Y, Liu F, & Xu Q. DNA binding, cleavage, and cytotoxic activity of the preorganized dinuclear zinc (II) complex of triazacyclononane derivatives. Biocon Chem. 2008; 19(2):490–498.
Zhang Y.-P, Ma Z.-Y., Gao C.-Y., Qiao X, Tian J.-L., Gu W, Liu X, Xu J.-Y., Zhao J.-Z. and Yan S.-P. Two dpa-based zinc (II) complexes as potential anticancer agents: nuclease activity, cytotoxicity and apoptosis studies. New J Chem. 2016; 40:7513–7521.
An Y, Lin YY, Wang H, Sun HZ, Tong ML, Ji LN, & Mao ZW. Cleavage of double-strand DNA by zinc complexes of dicationic 2,2′-dipyridyl derivatives. Dalton trans. 2007; 12:1250–1254.
Fahmy HM, Mosleh AM, El-Sayed AA, & El-Sherif AA. Novel palladium (II) and Zinc (II) Schiff base complexes: Synthesis, biophysical studies, and anticancer activity investigation. J Innov Technol Res. 2023;79:127236.
Niu M. Hong G, Chang X, Li Z, Li A. comparative study of cytotoxicity and interaction with DNA/protein of five transition metal complexes with Schiff base ligands. J Photochem Photobiol. B Biol., 2015;148:232–241.
Hassan N, El-Sonbati AZ, El-Desouky MG. Synthesis, characterization, molecular docking and DNA binding studies of Cu (II), Ni (II), Zn (II) and Mn (II) complexes. J Mol Liq. 2017; 242:293–307.
Lavanya M, Haribabu J, Ramaiah K, Yadav CS, Chitumalla RK, Jang J, Karvembu R, Reddy AV, Jagadeesh M. 2′- Thiophenecarboxaldehyde derived thiosemicarbazone metal complexes of copper (II), palladium (II) and zinc (II) ions: synthesis, spectroscopic characterization, anticancer activity and DNA binding studies, Inorg. Chim Acta. 2021;524:120440.
Kumar Y, Singh NK, Singh VD, Ali I, Tiwari RK, Kumar A, Pandey DS. DNA/Protein binding and anticancer activity of Zn (II) complexes based on azo- Schiff base ligands, Inorg. Chim Acta. 2022;538:120963.

Published: Nov 3, 2025
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
