Tamanna Kaundal
Panjab University Research Centre, Goswami Ganesh Dutta Sanatan Dharma College (GGDSD) College, Department of Biotechnology, Sector-32, Chandigarh, India
Navneet Batra
Panjab University Research Centre, Goswami Ganesh Dutta Sanatan Dharma College (GGDSD) College, Department of Biotechnology, Sector-32, Chandigarh, India
Anjali Sharma
DAV College, Department of Biotechnology, Sector- 10, Chandigarh, India
DOI: https://doi.org/10.14456/apst.2025.51
Keywords: Antibiofilm activity Antimicrobial activity Biosurfactants Lactic acid bacteria Pediococcous pentosaceus
Abstract
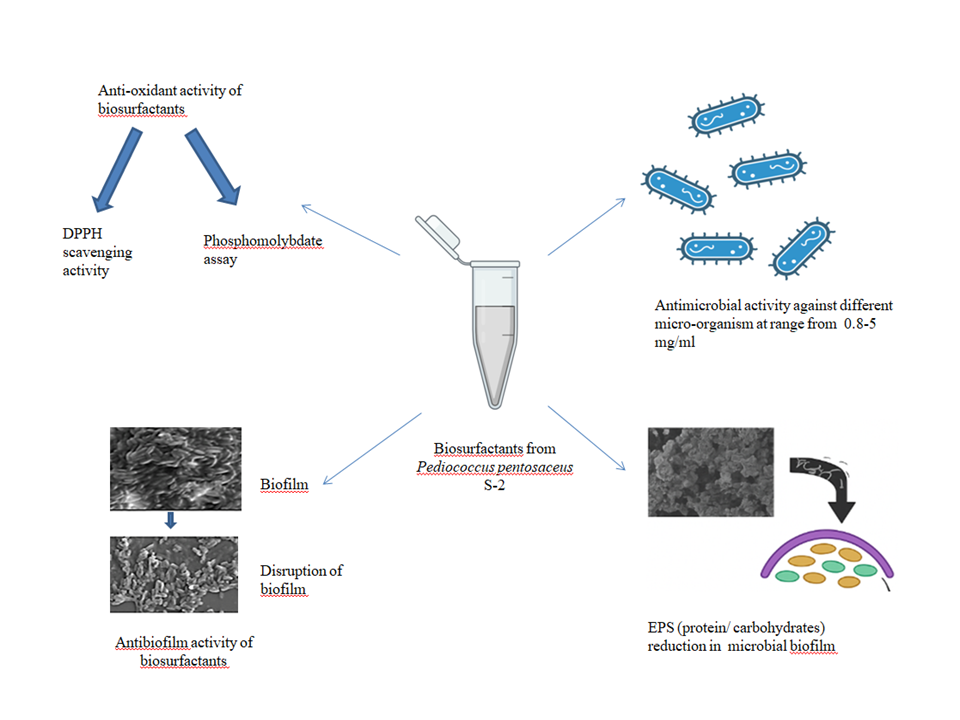
Numerous Lactic acid bacteria (LAB) tend to produce different types of biosurfactants i.e surface-active compounds. In the current investigation, biosurfactants derived from Pediococcus pentosaceus S-2 were explored for anti-oxidant, antimicrobial and antibiofilm activities against different test microorganisms. Pediococcus pentosaceus S-2 derived biosurfactants exhibited radical scavenging activity and antioxidant activity of 71% at a concentration of 600 µg. Antimicrobial activity was observed against different micro-organisms in the range between 0.8-5 mg/mL. At 4X minimum inhibitory concentration (MIC), there was 59-65 % inhibition of microbial biofilms in the presence of biosurfactants. Additionally, search engine marketing (SEM) microscopy provided additional evidence of the dispersion of different microbial biofilms in the presence of biosurfactants. Moreover, the biosurfactants also led to a reduction in the content of extracellular polymeric substances (EPSs), including proteins and carbohydrates, further contributing to the mitigation of microbial biofilms. These findings underscore the multifaceted potential of biosurfactants derived from Pediococcus pentosaceus S-2, suggesting their promising role as alternatives to synthetic surfactants across various applications, including antimicrobial, antibiofilm, and antioxidant functions.
How to Cite
Kaundal, T., Batra, N., & Sharma, A. (2025). Evaluation of the anti-oxidant, anti-microbial, antibiofilm potential of biosurfactants derived from Pediococcus pentosaceus S-2. Asia-Pacific Journal of Science and Technology, 30(04), APST–30. https://doi.org/10.14456/apst.2025.51
References
Gaspar P, Carvalho AL, Vinga S, Santos H, Neves AR. From physiology to systems metabolic engineering for the production of biochemicals by lactic acid bacteria. Biotechnol Adv. 2013;31(6):764–88.
Bintsis T. Lactic acid bacteria: their applications in foods. J Bacteriol Mycol. 2018;6(2):89–94.
Hajfarajollah H, Eslami P, Mokhtarani B, Akbari Noghabi K. Biosurfactants from probiotic bacteria: A review. Biotechnol Appl Biochem. 2018;65(6):768–83.
Nayarisseri A, Singh P, Singh SK. Screening, isolation and characterization of biosurfactant producing Bacillus subtilis strain ANSKLAB03. Bioinformation. 2018;14(6):304–314.
Santos DK, Rufino RD, Luna JM, Santos VA, Sarubbo LA. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int J Mol Sci. 2016;17(3):401.
Sarubbo LA, Silva MG, Durval IJB, Bezerra KGO, Ribeiro BG, Silva IA, Twigg MS, Banat IM. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem Eng J. 2022; 181:108377.
Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295(5559):1487.
Davey ME, O’Toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev. 2000;64(4):847-867.
Banat IM, Satpute SK, Cameotra SS, Patil R, Nyayanit NV. Cost effective technologies and renewable substrates for biosurfactants’ production. Front Microbiol. 2014; 5:697.
Kumar A, Singh SK, Kant C, Verma H, Kumar D, Singh PP, Modi A, Droby S, Kesawat MS, Alavilli H, Bhatia SK, Saratale GD, Saratale RG, Chung SM, Kumar M. Microbial biosurfactant: A New Frontier for sustainable agriculture and pharmaceutical industries. Antioxidants (Basel). 2021;10(9):1472.
Bjerk TR, Severino P, Jain S, Marques C, Silva AM, Pashirova T, Souto EB. Biosurfactants: Properties and applications in drug delivery, biotechnology and ecotoxicology. Bioengineering (Basel). 2021;8(8):115.
Kaundal T, Sharma A, Batra N. Isolation of Biosurfactant Producing Pediococcus pentosacaeus from Laboratory Controlled (Simulated) Fermentation of Indian Wheat-based Seera. Curr J Appl Sci Technol. 2022;41(26):12–23.
Kanwar SS, Bhushan K. Ethnic Fermented Foods and Beverages of Himachal Pradesh. In: Tamang J, editor. Ethnic fermented foods and beverages of india: Science history and culture. Singapore: Springer; 2020.
Savitri, Thakur N, Kumar D, Bhalla TC. Microbiological and biochemical characterization of Seera: A traditional fermented food of Himachal Pradesh. Int J Food Ferment Technol. 2012;2(1):49–56.
Augustin M, Majeste P, Hippolyte M, Leopold T. Effect of Biosurfactants Extracted from a locally fermented milk (Pendidam) on Its Shelf Life. J Adv Biol Biotechnol. 2015;3(1):12–22.
Satpute S, Mone N, Das P, Banpurkar A, Banat I. Lactobacillus acidophilus Derived Biosurfactant as a Biofilm Inhibitor: A promising investigation using microfluidic approach. Appl Sci. 2018;8(9):1555.
Baliyan S, Mukherjee R, Priyadarshini A, Vibhuti A, Gupta A, Pandey RP, et al. Determination of Antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules. 2022;27(4):1326.
Khatoon M, Islam E, Islam R, Rahman AA, Alam AHMK, Khondkar P, et al. Estimation of total phenol and in vitro antioxidant activity of Albizia procera leaves. BMC Res Notes. 2013;6(1):121.
Magaldi S, Mata-Essayag S, Hartung C. Well diffusion for antifungal susceptibility testing. Int J Infect Dis. 2004; 8:39–45.
Kahlmeter G, Brown D, Goldstein F, Macgowan A, Mouton J, Odenholt I, et al. European committee on antimicrobial susceptibility testing (EUCAST) technical notes on antimicrobial susceptibility testing. Clin Microbiol Infect. 2006; 12:501–503.
Yan X, Gu S, Cui X, Shi Y, Wen S, Chen H, Ge J. Antimicrobial, anti-adhesive and anti-biofilm potential of biosurfactants isolated from Pediococcus acidilactici and Lactobacillus plantarum against Staphylococcus aureus CMCC26003. Microb Pathog. 2019; 127:12–20.
Wilson C, Lukowicz R, Merchant S, Valquier-Flynn H, Caballero J, Sandoval J, et al. Quantitative and qualitative assessment methods for biofilm growth: A Mini-review. Res Rev J Eng Technol. 2017;6(4):1-12.
Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthone on the auto oxidation of soybean in cyclodextrin emulsion. J Agric Food Chem. 1992;40(1):945–948.
Giri S, Ryu E, Sukumaran V, Park S. Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microb Pathog. 2019; 132:66–72.
Jemil N, Ayed HB, Manresa A, Nasri M, Hmidet N. Antioxidant properties, antimicrobial and anti-adhesive activities of DCS1 lipopeptides from Bacillus methylotrophicus DCS1. BMC Microbiol. 2017;17(1):144.
Bardone E, Marzocchella A, Bravi M, Silva I, Gercyane K, Bezerra O, et al. Evaluation of the emulsifying and antioxidant capacity of the biosurfactant produced by Candida bombicola URM 3718. Chem Eng Trans. 2020; 79:355–360.
Adnan M, Siddiqui AJ, Hamadou WS, Ashraf SA, Hassan MI, Snoussi M, et al. Functional and structural characterization of Pediococcus pentosaceus-derived biosurfactant and its biomedical potential against bacterial adhesion, quorum sensing, and biofilm formation. Antibiotics (Basel). 2021;10(11):1371.
Jenny K, Kappeli O, Fiechter A. Biosurfactant from Bacillus licheniformis: structural analysis and characterization. Appl Microbiol Biotechnol. 1991;36(1):5–13.
Lotfabad TB, Abassi H, Ahmadkhaniha R, Roostaazad F, Masoomi HS, Zahiri G, et al. Structural characterization of a rhamnolipid-type biosurfactant produced by Pseudomonas aeruginosa MR01: Enhancement of di-rhamnolipid proportion using gamma irradiation. Colloids Surf B Biointerfaces. 2011;81(2):397–405.
Adnan M, Siddiqui AJ, Hamadou WS, Ashraf SA, Hassan MI, Snoussi M, et al. Functional and structural characterization of Pediococcus pentosaceus-derived biosurfactant and its biomedical potential against bacterial adhesion, quorum sensing, and biofilm formation. Antibiotics. 2021;10(11):1371.
Siddiqui AJ, Hamadou WS, Surti M, Awadelkareem AM, Ashraf SA, et al. Inhibition of bacterial adhesion and antibiofilm activities of a glycolipid biosurfactant from Lactobacillus rhamnosus with its physicochemical and functional properties. Antibiotics (Basel). 2021;10(12):1546.
Rodrigues L, Teixeira JA, Oliveira R. Biosurfactant from Lactococcus lactis 53 inhibits microbial adhesion on silicone rubber. Appl Microbiol Biotechnol. 2004;66(3):306–311.
Gudiña EJ, Teixeira JA, Rodrigues LR. Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloids Surf B Biointerfaces. 2010;76(1):298–304.
Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–633.
Adnan M, Siddiqui AJ, Hamadou WS, Ashraf SA, Hassan MI, Snoussi M, et al. Functional and structural characterization of Pediococcus pentosaceus-derived biosurfactant and its biomedical potential against bacterial adhesion, quorum sensing, and biofilm formation. Antibiotics (Basel). 2021;10(11):1371.
Kim LH, Jung Y, Kim SJ, Kim CM, Yu HW, Park HD, et al. Use of rhamnolipid biosurfactant for membrane biofouling prevention and cleaning. Biofouling. 2021;31(2):211–220.

Published:
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
