Muhammad F. Arif
Laboratory of Biology, Department of Biology, Faculty of Mathematics and Life Sciences, Mulawarman University, Samarinda, 75242, Indonesia
https://orcid.org/0000-0001-9964-1294
Chalvia Zuyyina
Laboratory of Genetics and Breeding, Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia
https://orcid.org/0009-0004-6494-1237
Rina S. Kasiamdari
Laboratory of Plant Systematics, Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia
https://orcid.org/0000-0003-4125-1490
Ani Widiastuti
Laboratory of Integrated Pest Control, Faculty of Agriculture, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia
https://orcid.org/0000-0001-6745-5614
Ganies R. Aristya
Laboratory of Genetics and Breeding, Faculty of Biology, Universitas Gadjah Mada, Yogyakarta, 55281, Indonesia
https://orcid.org/0000-0001-9251-5076
DOI: https://doi.org/10.14456/apst.2025.78
Keywords: FaCHS gene FaPYR1 gene Fragaria × ananassa Fruit ripening indole-3-acetic acid
Abstract
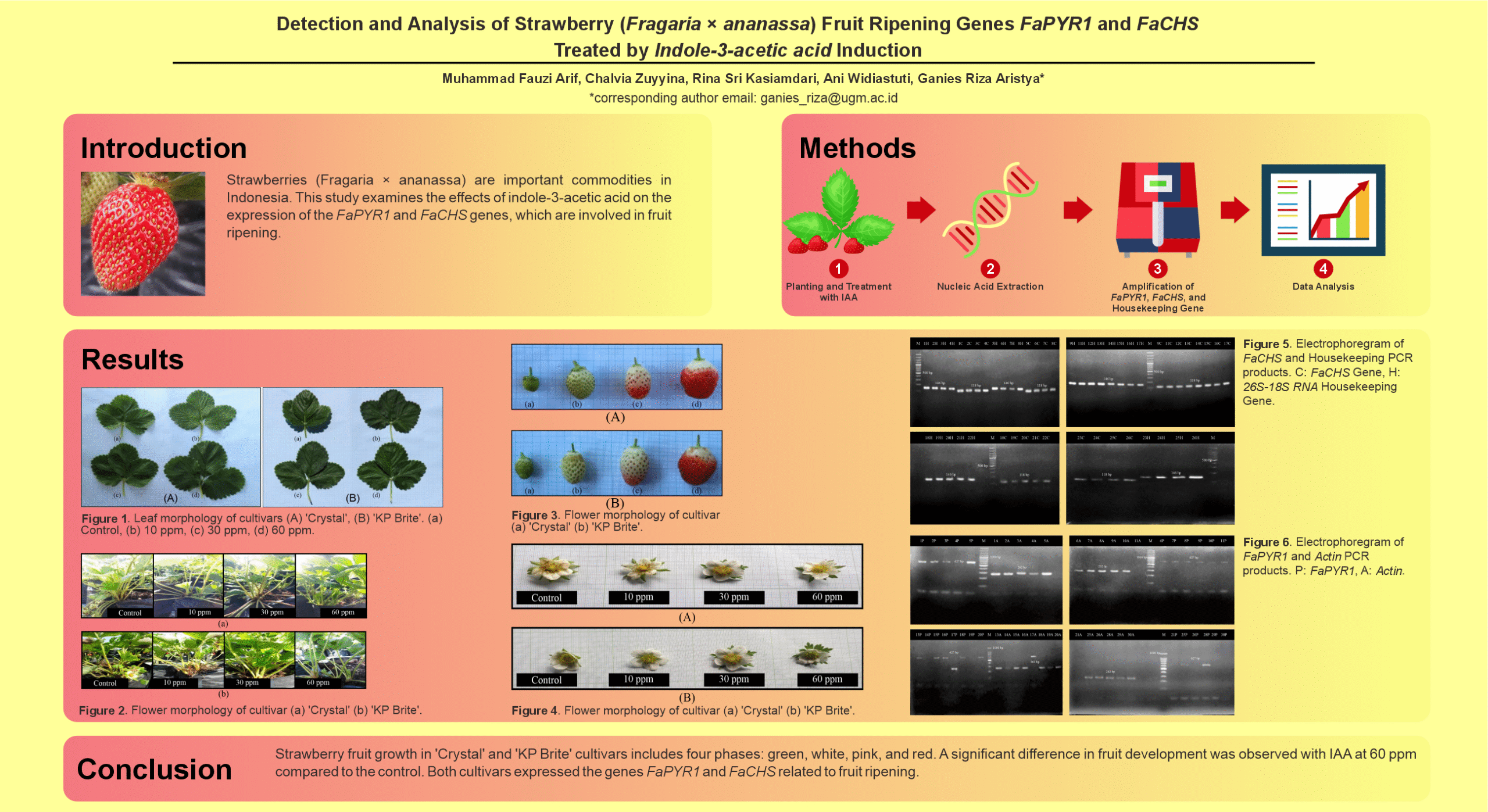
Strawberries (Fragaria × ananassa) are economically significant commodities in Indonesia, where ripeness influences their quality and marketability. This study investigates the role of the FaPYR1 and FaCHS genes, involved in fruit ripening, in strawberries treated with varying concentrations of indole-3-acetic acid (IAA). A key research gap lies in understanding how IAA affects these gene expressions and strawberry morphology. Strawberry plants were treated with 10 ppm, 30 ppm, and 60 ppm of IAA, and their genomic deoxyribonucleic acid (DNA) was analyzed using polymerase chain reaction (PCR), alongside morphological evaluations. Results revealed that 30 ppm of IAA significantly enhanced leaf dimensions and fruit genomic DNA concentration during the red stage. Conversely, the highest leaf count was observed at 60 ppm, while control plants exhibited the lowest morphological and genomic outcomes. PCR analysis confirmed FaCHS gene expression across all treatments, but FaPYR1 expression was inconsistent. These findings highlight the potential of IAA, particularly at 30 ppm, to optimize strawberry growth and ripening, offering valuable insights for improving cultivation practices and fruit quality management.
How to Cite
Arif, M. F., Zuyyina, C., Kasiamdari, R. S., Widiastuti, A., & Aristya, G. R. (2025). Detection and analysis of strawberry (Fragaria × ananassa) fruit ripening Genes FaPYR1 and FaCHS treated by Indole-3-acetic acid induction . Asia-Pacific Journal of Science and Technology, 30(05), APST–30. https://doi.org/10.14456/apst.2025.78
References
Hancock JF. Temperate fruit crop breeding: Germplasm Genomics. USA. Springer. 2008; 14:1-15.
M. Deák Sz, Márk GI, Szabó T, Füleky Gy. Spectral properties of strawberry plants. Int J Hortic Sci. 2007;13(2):17–22.
Hummer KE, Janick J. Rosaceae: Taxonomy, Economic Importance, Genomics. Genetics and genomics of Rosaceae. New York. Springer. 2009; 6:1-17.
Edger PP, Poorten TJ, VanBuren R, Hardigan MA, Colle M, McKain MR, et al. Origin and evolution of the octoploid strawberry genome. Nat Genet. 2019; 51:541–547.
Malhotra KS. Horticultural Production Statistics: Directorate General of Horticulture. In: Ministry of Agriculture. Jakarta; 2015.
Hanif Z, Ashari H. Factors affecting the development of strawberry in Indonesia. J Interam Soc Trop Hortic. 2013; 1:151–155.
Fecka I, Nowicka A, Kucharska AZ, Sokół-Łętowska A. The effect of strawberry ripeness on the content of polyphenols, cinnamates, L-ascorbic and carboxylic acids. J Food Compos Anal. 2021; 95:1–9.
Bao X, Jia Y, Liu X, Zhu Y, Tong Y, Wang Y, et al. Transcriptome comparison of strawberry ripen fruit harvested/collected from plants grown under three different temperature treatments. Ann Agric Crop Sci. 2023;8(3):1–8.
Taiz L, Zeiger E. Plant Physiology 5th Edition. Sunderland: Sinauer Associates; 2010.
Symons GM, Chua YJ, Ross JJ, Quittenden LJ, Davies NW, Reid JB. Hormonal changes during non-climacteric ripening in strawberry. J Exp Bot. 2012;63(13):4741–4750.
Sangiorgio D, Cellini A, Spinelli F, Donati I. Promoting strawberry (Fragaria ananassa) stress resistance, growth, and yield using native bacterial biostimulants. Agronomy. 2023; 13:1–12.
Li T, Dai Z, Zeng B, Li J, Ouyang J, Kang L, et al. Autocatalytic biosynthesis of abscisic acid and its synergistic action with auxin to regulate strawberry fruit ripening. Hortic Res. 2022; 9:1–11.
Chai YM, Jia HF, Li CL, Dong QH, Shen YY. FaPYR1 is involved in strawberry fruit ripening. J Exp Bot. 2011; 62:5079–5089.
Davies C, Boss PK, Robinson SP. Treatment of grape berries, a nonclimacteric fruit with a synthetic auxin, retards ripening and alters the expression of developmentally regulated genes. Plant Physiol. 1997;115(3):1155–1161.
Ban T, Ishimaru M, Kobayashi S, Shiozaki S, Goto-Yamamoto N, Horiuchi S. Abscisic acid and 2,4-dichlorophenoxyacetic acid affect the expression of anthocyanin biosynthetic pathway genes in Kyoho grape berries. J Hortic Sci Biotechnol.2003;78(4):586–589.
Teribia N, Tijero V, Munné-Bosch S. Linking hormonal profiles with variations in sugar and anthocyanin contents during the natural development and ripening of sweet cherries. Biotechnol. 2016; 33:824–833.
He P, Ma Y, Dai H, Li L, Liu Y, Li H, et al. Characterization of the hormone and stress-induced expression of FaRE1 retrotransposon promoter in strawberry. J Plant Biol. 2012; 55:1–7.
Ayub RA, Bosetto L, Galvão CW, Etto RM, Inaba J, Lopes PZ. Abscisic acid involvement on expression of related gene and phytochemicals during ripening in strawberry fruit Fragaria ananassa cv. Camino Real Sci Hortic. 2016; 203:178–184.
Muñoz C, Hoffmann T, Escobar NM, Ludemann F, Botella MA, Valpuesta V, et al. The strawberry fruit fra a allergen functions in flavonoid biosynthesis. Mol Plant. 2010; 3:113–124.
Sun Y, Yang Y, Yuan Z, Muller JL, Yu C, Xu Y, et al. Overexpression of the Arabidopsis gene upright rosette reveals a homeostatic control for indole-3-acetic acid. Plant Physiol. 2010;153(3):1311–1320.
Zhao Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-Acetic acid in plants. Mol Plant. 2012; 5(2):334–338.
Aoi Y, Tanaka K, Cook SD, Hayashi KI, Kasahara H. GH3 auxin-amido synthetases alter the ratio of indole-3-acetic acid and phenylacetic acid in Arabidopsis. Plant Cell Physiol. 2020; 61:596–605.
Kriechbaumer V, Park WJ, Piotrowski M, Meeley RB, Gierl A, Glawischnig E. Maize nitrilases have a dual role in auxin homeostasis and β-cyanoalanine hydrolysis. J Exp Bot. 2007; 58:4225–4233.
Kasahara H. Current aspects of auxin biosynthesis in plants. Biosci Biotechnol Biochem. 2016; 80:34–42.
De Souza Vandenberghe LP, Rodrigues C, De Oliveira J, Soccol CR. Biotransformation of Waste Biomass into High Value Biochemicals. New York. Springer. 2014.
Zhao Y. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol. 2010; 61:49–64.
Hussein S, Ling APK, Ng TH, Ibrahim R, Paek KY. Adventitious roots induction of recalcitrant tropical woody plant, Eurycoma longifolia. Rom Biotechnol Lett. 2012; 17:7026–7035.
Yang T, Davies PJ. Promotion of stem elongation by indole-3-butyric acid in intact plants of Pisum sativum L. Plant Growth Regul. 1999; 27:157–160.
Southwick SM, Poovaiah BW. Auxin movement in strawberry fruit corresponds to its growth-promoting activity. J Am Soc Hortic Sci. 1987; 112:139–142.
Vlahos JC, Dragassaki M, Vasilaki A, Assargiotaki I, Tsatsakis AM. The effect of slow-release polymeric derivatives of auxins (NAA and 2,4-D) on regeneration of achimenes in vitro. Hort Science. 1995;31(4):340–345.
Uggla C, Moritz T, Sandberg G, Sundberg B. Auxin as a positional signal in pattern formation in plants. Proc Natl Acad Sci U S A. 1996; 93:9282–9286.
De Zio E, Trupiano D, Karady M, Antoniadi I, Montagnoli A, Terzaghi M. Tissue-specific hormone profiles from woody poplar roots under bending stress. Physiol Plant. 2019; 165:101–113.
Yang T, Davies PJ, Reid JB. Genetic dissection of the relative roles of auxin and gibberellin in the regulation of stem elongation in intact light-grown peas. Plant Physiol. 1996; 110:1029–1034.
Durachko DM, Cosgrove DJ. Measuring plant cell wall extension (creep) induced by acidic pH and by alpha-expansin. J Vis Exp. 2009; 25:1–4.
Wodzicki TJ, Rakowski K, Starck Z, Porandowski J, Zajączkowski S. Apical control of xylem formation in the pine stem. I. Auxin effects and distribution of assimilates. Acta Soc Bot Pol. 1982; 51(2):187–201.
Chen JG, Ullah H, Young JC, Sussman MR, Jones AM. ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev. 2001; 15:902–911.
Pacheco-Villalobos D, Díaz-Moreno SM, van der Schuren A, Tamaki T, Kang YH, Gujas B, et al. The effects of high steady state auxin levels on root cell elongation in Brachypodium. Plant Cell. 2016; 28:1009–1024.
Danial GH, Ibrahim DA, Omer MS. Response of running shoot tips of strawberry (Fragaria x ananasa) for in vitro propagation in Kurdistan Region of Iraq. Int J Environ Agric Biotechnol. 2016; 1:164–169.
Nehra NS, Stushnoff C, Kartha KK. Direct shoot regeneration from strawberry leaf disks. J Am Soc Hortic Sci. 2022; 114:1014–1018.
Liu ZR, Sanford JC. Plant regeneration by organogenesis from strawberry leaf and runner tissue. Hort Science. 2022; 23:1057–1059.
Jia H, Jiu S, Zhang C, Wang C, Tariq P, Liu Z, et al. Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor. Plant Biotechnol J. 2016; 14:2045–2065.
Chen J, Mao L, Lu W, Ying T, Luo Z. Transcriptome profiling of postharvest strawberry fruit in response to exogenous auxin and abscisic acid. Planta. 2016; 243:183–197.
Luo J, Zhou JJ, Zhang JZ. Aux/IAA gene family in plants: Molecular structure, regulation, and function. Int J Mol Sci. 2018; 19:1–17.
Liu C, Liu W, Lu X, Ma F, Chen W, Yang J, et al. Application of multispectral imaging to determine quality attributes and ripeness stage in strawberry fruit. PLoS One. 2014; 9(2):1–8.
Janurianti NMD, I Made Supartha Utama, Ida Bagus Wayan Gunam. Colour and quality of strawberry fruit (Fragaria x ananassa Duch.) at different levels of maturity. SEAS. 2021; 5:22–28.
Elhariri E, El-Bendary N, Mahmoud Saleh S. Strawberry-DS: Dataset of annotated strawberry fruits images with various developmental stages. Data Brief. 2023; 48:1–9.
Kim J, Lee JG, Hong Y, Lee EJ. Analysis of eight phytohormone concentrations, expression levels of ABA biosynthesis genes, and ripening-related transcription factors during fruit development in strawberry. J Plant Physiol. 2019; 239:52–60.
Mao W, Han Y, Chen Y, Sun M, Feng Q, Li L, et al. Low temperature inhibits anthocyanin accumulation in strawberry fruit by activating FvMAPK3-induced phosphorylation of FvMYB10 and degradation of Chalcone Synthase 1. Plant Cell. 2022; 34:1226–1249.
Taghavi T, Patel H, Rafie R. Comparing pH differential and methanol-based methods for anthocyanin assessments of strawberries. Food Sci Nutr. 2022; 10:2123–2131.
Peng Y, Jiang Y, He C, She M, Li M, Chen Q, et al. Exogenous GR24 inhibits strawberry tillering by affecting the phytohormone signaling and sugar metabolism pathways. Agronomy. 2023; 13:1–21.
Guo J, Wang S, Yu X, Dong R, Li Y, Mei X, et al. Polyamines regulate strawberry fruit ripening by abscisic acid, auxin, and ethylene. Plant Physiol. 2018; 177:339–351.
Liu DJ, Chen JY, Lu WJ. Expression and regulation of the early auxin-responsive Aux/IAA genes during strawberry fruit development. Mol Biol Rep. 2011; 38:1187–1193.
Archbold DD, Dennis FG. Quantification of free ABA and free and conjugated IAA in strawberry achene and receptacle tissue during fruit development. J Am Soc Hortic Sci. 2022; 109:330–335.
Zhang X, Liu Y, Liu Q, Zong B, Yuan X, Sun H, et al. Nitric oxide is involved in abscisic acid-induced photosynthesis and antioxidant system of tall fescue seedlings response to low-light stress. Environ Exp Bot. 2018; 155:226–238.
Li C, Hou B. Molecular mechanism of abscisic acid in regulating fruit ripening. J Fruit Sci. 2023; 40:1-10.
Li Y, Hu J, Xiao J, Guo G, Jeong BR. Foliar thidiazuron promotes the growth of axillary buds in Strawberry. Agronomy. 2021; 11:1–11.
Aristya GR, Dyatama GR, Zuyyina C, Maulina NTA, Musthofa A, Arif MF, et al. Relationship analysis of FaPYR1 and FaCHS genes encoding fruit ripening of three species of strawberries (Fragaria spp.) fruit. Biodiversitas. 2023; 24:87–97.
Muñoz C, Sánchez-Sevilla JF, Botella MA, Hoffmann T, Schwab W, Valpuesta V. Polyphenol composition in the ripe fruits of Fragaria species and transcriptional analyses of key genes in the pathway. J Agric Food Chem. 2011; 59:12598–12604.
Lin Y, Zhang L, Zhang J, Zhang Y, Wang Y, Chen Q, et al. Identification of anthocyanins-related glutathione s-transferase (Gst) genes in the genome of cultivated strawberry (Fragaria × ananassa). Int J Mol Sci. 2020; 21:1–19.
Wang S, Song M, Guo J, Huang Y, Zhang F, Xu C, et al. The potassium channel FaTPK1 plays a critical role in fruit quality formation in strawberry (Fragaria × ananassa). Plant Biotechnol J. 2018; 16:737–748.
Lu W, Chen J, Ren X, Yuan J, Han X, Mao L, et al. One novel strawberry MADS-box transcription factor FaMADS1a acts as a negative regulator in fruit ripening. Sci Hortic. 2018; 227:124–131.
Jia KN, Wei YB, Wang Y. Responses of Mimosa pudica and Fragaria vesca to electrical pulse stimulation. Zhiwu Shengli Xuebao/Plant Physiol J. 2017; 53:1–8.

Published:
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
