Lotis M. Balala
College of Veterinary Medicine, Visayas State University, Visca, Baybay City, Leyte, Philippines
Bernadette C. Mendoza
Institute of Biological Sciences, University of the Philippines Los Baños, College, Laguna, Philippines
Loinda R. Baldrias
College of Veterinary Medicine, University of the Philippines Los Baños, College, Laguna, Philippines
Joseph S. Masangkay
College of Veterinary Medicine, University of the Philippines Los Baños, College, Laguna, Philippines
DOI: https://doi.org/10.14456/apst.2025.3
Keywords: Culture ELISA IgG MDR Native chickens PCR Salmonella
Abstract
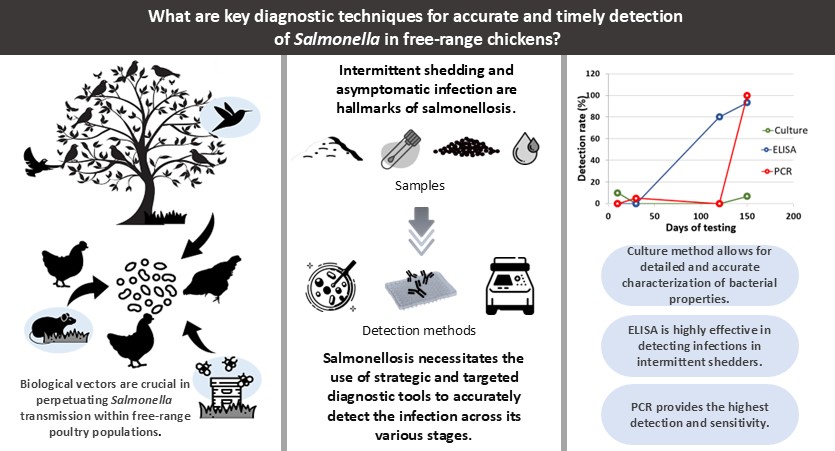
A compromise in biosecurity measures is a major determinant of pathogen emergence in free-range animals. This study aimed to detect antimicrobial-resistant Salmonella in free-range Philippine native chickens. Clinical and environmental samples were collected from a free-range farm with 50 Banaba x Paraoakan native chickens to analyze Salmonella using conventional culture, polymerase chain reaction (PCR), and enzyme-linked immunosorbent assay (ELISA). Antimicrobial resistance was confirmed through the agar disc diffusion method. Salmonella persisted in poultry samples from Day 10 to Day 150 with an overall detection rate of 8.9% (21/237). The earliest detection of Salmonella was on Day 10 by conventional culture and on Day 30 by PCR posing a detection rate of 2.11% (5/237) and 8.04% (16/199), respectively. ELISA detected seropositivity on Day 120 with an overall seroconversion rate of 60%. Antibiotic resistance of isolates was 80% in ampicillin, doxycycline, tetracycline, and trimethoprim-sulfamethoxazole; 60% in kanamycin and cefuroxime; and 40% in neomycin. Eighty percent (4/5) of the isolates demonstrated a potential multidrug resistance pattern. Multidrug resistant Salmonella persisted in free-range native chickens posing food quality and safety implications. Biosecurity and preharvest strategies should be improved to reduce the pathogen in free-range farming.
How to Cite
Balala, L. M., Mendoza, B. C., Baldrias, L. R., & Masangkay, J. S. (2024). Detection of multidrug-resistant Salmonella in native chickens by culture, polymerase chain reaction, and enzyme linked immunosorbent assay. Asia-Pacific Journal of Science and Technology, 30(01), APST–30. https://doi.org/10.14456/apst.2025.3
References
Ricke SC, Rothrock MJ Jr. Gastrointestinal microbiomes of broilers and layer hens in alternative production systems. Poult Sci. 2020;99(2):660–669.
Mollenhorst H, van Woudenbergh CJ, Bokkers EGM, de Boer IJM. Risk factors for Salmonella enteritidis infections in laying hens. Poult Sci. 2005;84(8):1308–1313.
Cui L, Liu Q, Jiang Z, Song Y, Yi S, Qiu J, et al. Characteristics of Salmonella from Chinese native chicken breeds fed on conventional or antibiotic-free diets. Front Vet Sci. 2021;8:607491.
Hedman HD, Vasco KA, Zhang L. A Review of Antimicrobial Resistance in Poultry Farming within Low-Resource Settings. Animals. 2020;10(8):1264.
International Standard. Microbiology of food and animal feeding stuffs — Horizontal method for the detection of Salmonella spp. 4th ed. ISO Office, Switzerland; 2002.
Rahn K, De Grandis SA, Clarke RC, McEwen SA, Galán JE, Ginocchio C, et al. Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol Cell Probes. 1992;6(4):271-279.
Beal RK, Powers C, Wigley P, Barrow PA, Smith AL. Temporal dynamics of the cellular, humoral and cytokine responses in chickens during primary and secondary infection with Salmonella enterica serovar Typhimurium. Avian Pathol. 2004;33(1):25-33.
Wigley P, Hulme SC, Powers C, Beal RK, Berchieri A Jr, Smith A, et al. Infection of the reproductive tract and eggs with Salmonella enterica serovar Pullorum in the chicken is associated with suppression of cellular immunity at sexual maturity. Infect Immun. 2005;73(5):2986-2990.
Clinical Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 28th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2018.
Learn-Han L, Yoke-Kqueen C, Shiran MS, Sabrina S, Noor Zaleha AS, Sim JH, et al. Molecular characterization and antimicrobial resistance profiling of Salmonella enterica subsp. enterica isolated from ‘Selom’ (Oenanthe stolonifera). Int Food Res. J. 2009;16(2):191-202.
Sweeney MT, Lubbers BV, Schwarz S, Watts JL. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J Antimicrob Chemother. 2018;73:1460–1463.
Sullivan G, Guo X, Tokman JI, Roof S, Trmcic A, Baker RC, et al. Extended enrichment procedures can be used to define false-negative probabilities for cultural gold standard methods for Salmonella detection, facilitating comparisons between gold standard and alternative methods. J Food Prot. 2020;83(6):1030-1037.
Wang M, Zhang Y, Tian F, Liu X, Du S, Ren G. Overview of rapid detection methods for Salmonella in foods: progress and challenges. Foods. 2021;10(10):2402.
Schrank IS, Mores MAZ, Costa JLA, Frazzon APG, Soncini R, Schrank A, et al. Influence of enrichment media and application of a PCR based method to detect Salmonella in poultry industry products and clinical samples. Vet Microbiol. 2010;82(1):45–53.
Oliveira GH, Berchieri A Jr, Montassier HJ. Chicken serologic response to Salmonella enterica serotype Typhimurium assessed by ELISA. Braz J Poult Sci. 2006;8(1):51-54.
Soria MC, Soria MA, Bueno DJ. Comparison of 2 culture methods and PCR assays for Salmonella detection in poultry feces. Poult Sci. 2012;91(3):616-626.
Carli KT, Unal GB, Gaiier V, Eyigor A. Detection of salmonellae in chicken feces by a combination of tetrathionate broth enrichment, capillary PCR, and capillary gel electrophoresis. J Clin Microbiol. 2001;39(5): 1871-1876.
European Committee on Antimicrobial Susceptibility Testing (EUCAST). New definitions of S, I and R from 2019 [Internet]. 2021 [cited 2022 September 20] Available from https://www.eucast.org/newsiandr/.
Balala LM, Rovira HG, Vizmanos MFC, Bernardo FAEM, Divina BP. Isolation, identification and antibiotic sensitivity testing of Salmonella spp. in chickens. Philipp J Vet Med. 2006;43(2): 63-70.
Thung TY, Mahyudin NA, Basri DF, Radzi WM, Nakaguchi Y, Nishibuchi M, et al. Prevalence and antibiotic resistance of Salmonella Enteritidis and Salmonella Typhimurium in raw chicken meat at retail markets in Malaysia. Poult Sci. 2016;95(8):1888-1893.
Abunna SF, Bedasa M, Beyene T, Ayana D, Mamo B, Duguma, R. Salmonella: Isolation and antimicrobial susceptibility tests on isolates collected from poultry farms in and around Modjo, Central Oromia, and Ethiopia. J animal poult sci. 2017;5(2):21-35.
Samanta I, Joardar SN, Das PK, Sar SK, Bandyopadhyay S, Dutta TK, et al. Prevalence and antibiotic resistance profiles of Salmonella serotypes isolated from backyard poultry flocks in West Bengal, India. J Appl Poult Res. 2014;23:536–545.
Bautista VALM, Mendoza BC. Multiple drug resistance profile of Salmonella enterica subsp. enterica serovar Kentucky obtained from apparently healthy layer chickens in San Jose, Batangas, Philipp J Vet Med. 2016:53(1):17-25.
Castro-Vargas RE, Herrera-Sánchez MP, Rodríguez-Hernández R, Rondón-Barragán IS. Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Vet World. 2020;13(10):2070-2084.
El Sharkawy H, Tahoun A, El-Gohary AEA, El-Abasy M, El-Khayat F, Gillespie T, et al. Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut Pathog. 2017;9(1):8.
Zhao X, Gao Y, Ye C, Yang L, Wang T, Chang W. Prevalence and characteristics of Salmonella isolated from free-range chickens in Shandong province, China. Biomed Res Int. 2016;2016(1):8183931.
Pham T, Ziora ZM, Blaskovich M. Quinolone antibiotics. Medchemcomm. 2019;10(10):1719–1739.
Karabasanavar NS, Madhavaprasad CB, Gopalakrishna SA, Hiremath J, Patil GS, Barbuddhe SB. Prevalence of Salmonella serotypes S. Enteritidis and S. Typhimurium in poultry and poultry products. J Food Saf. 2020;40(6):e12852.
Menashe O, Kaganskaya E, Baasov T, Yaron S. Aminoglycosides affect intracellular Salmonella enterica serovars typhimurium and virchow. Antimicrob Agents Chemother. 2008;52(3):920–926.
Mandal S, Manda MD, Pal NK. In vitro activity of gentamicin and amikacin against Salmonella enterica serovar Typhi: a search for a treatment regimen for typhoid fever. East Mediterr Health J. 2009;15(2):264-268.
Van Immerseel F, de Buck J, Pasmans IF, Bohez L, Boyen F, Haesbrouck F, et al. Intermittent long-term shedding and induction of carrier birds after infection of chickens early posthatch with low or high dose of Salmonella enteritidis. Poult Sci. 2004;83(11):1911-1916.
Umali DV, Lapuz RRSP, Suzuki T, Shirota J, Katoh H. Transmission and shedding patterns of Salmonella in naturally infectied captive wild roof rats (Rattus rattus) from a Salmonella-contaminated farm. Avian Dis. 2012;56:288-294.
Henzler DJ, Opitz HM, Saeed AM. Role of rodents in the epidemiology of Salmonella enterica serovar enteritidis and other Salmonella serovars in poultry farms. Salmonella enterica serovar enteritidis in humans and animals: Epidemiology, pathogenesis and control. Ames (USA): Iowa State University Press; 1999. p. 443.
Maciel BM, Sriranganathan N, Romano CC, dos Santos TF, Dias JC, Gross E, et al. Infection cycle of Salmonella enterica serovar Enteritidis in latent carrier mice. Can J Microbiol. 2012;58:1389-1395.
Gast RK, Mitchell BW, Holt PS. Airborne transmission of Salmonella enteritidis infection between groups of chicks in controlled-environment isolation cabinets. Avian Dis. 1998;42(2):315-320.
McWhorter AR, Chousalkar K. A long-term efficacy trial of a live, attenuated Salmonella typhimurium vaccine in layer hens. Front Microbiol. 2018;9:1380.
Zhuang M, Achmon Y, Cao Y, Liang X, Chen L, Wang H, et al. Distribution of antibiotic resistance genes in the environment. Environ Pollut. 2021;285:117402.

Published:
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
