Siu Hua Chang
Waste Management and Resource Recovery (WeResCue) Group, Faculty of Chemical Engineering, Universiti Teknologi MARA, Cawangan Pulau Pinang, 13500 Permatang Pauh, Pulau Pinang, Malaysia.
Siti Fatimah Abdul Halim
Waste Management and Resource Recovery (WeResCue) Group, Faculty of Chemical Engineering, Universiti Teknologi MARA, Cawangan Pulau Pinang, 13500 Permatang Pauh, Pulau Pinang, Malaysia.
Muhammad Ikram Abdul Halim
School of Industrial Technology, Universiti Sains Malaysia, 11800 Pulau Pinang, Malaysia.
Norhashimah Morad
School of Industrial Technology, Universiti Sains Malaysia, 11800 Pulau Pinang, Malaysia.
Keywords: copper green organic solvent palm kernel fatty acid distillate solvent extraction selectivity
Abstract
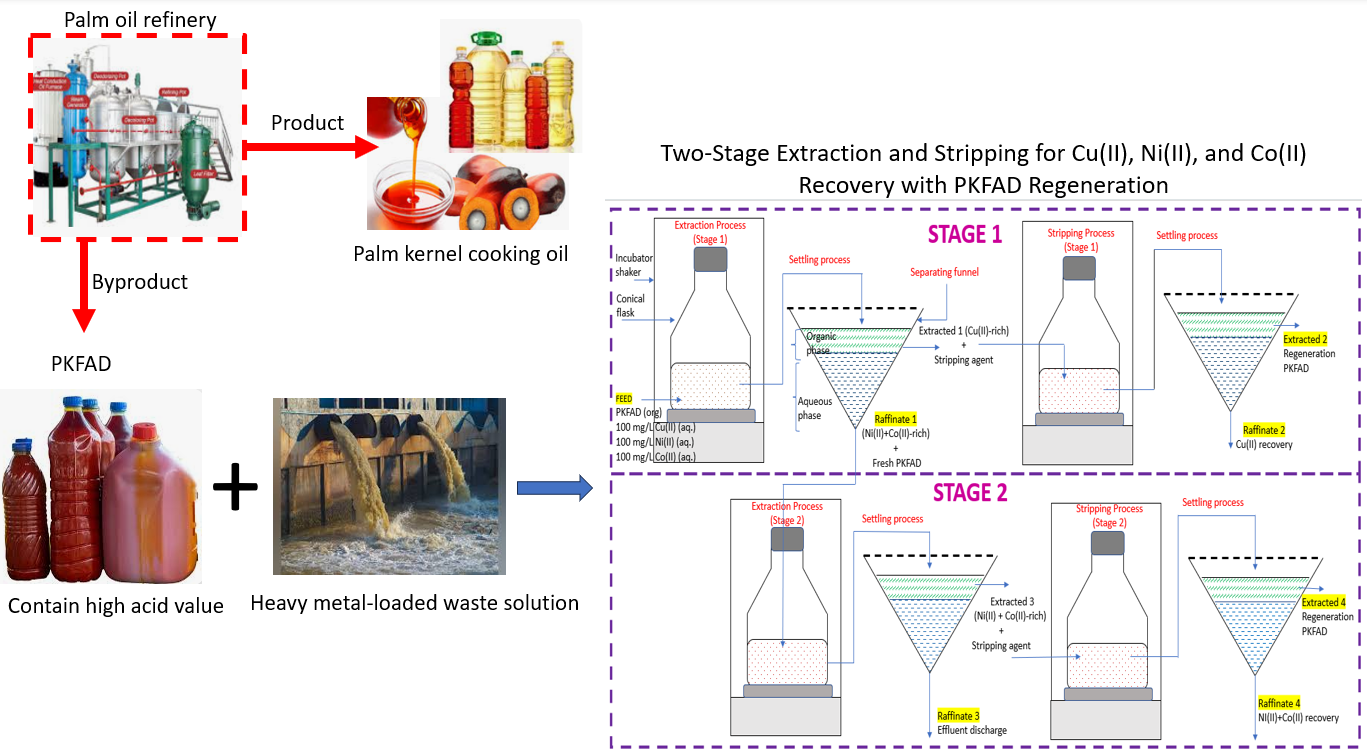
The objective of this study was to achieve the selective recovery of Cu(II) from aqueous solutions in the presence of Ni(II) and Co(II) by utilising a green approach, palm kernel fatty acid distillate (PKFAD) as the organic phase. PKFAD was employed without the use of diluent, extractant, or modifier typically required in conventional solvent extraction processes. In the first stage of the procedure, Cu(II) was selectively extracted and stripped from the multi-element solution. In the second stage, Ni(II) and Co(II) were subsequently recovered. The selectivity of the extraction process was driven by the difference in pH-dependence equilibrium (pHeq) for each metal ion. A high Cu(II) efficiency of 97% was achieved in the first stage at pHeq of 4.8, while Ni(II) and Co(II) extraction efficiencies of 88% and 85%, respectively, were obtained in the subsequent extraction stage at a pHeq of 5.9. Separation factor for Cu(II) over Ni(II) and Co(II) were ≥ 180, indicating effective selective separation. Additionally, the PKFAD was successfully regenerated, with the stripping process achieved 98% of Cu(II), 85% of Ni(II) and 72% of Co(II) recovery. These results highlight the efficacy of PKFAD as a sustainable, regenerable organic phase with high potential for selective metal ion recovery.
How to Cite
Chang, S. H. ., Abdul Halim, S. F. ., Abdul Halim, M. I. ., & Morad, N. . (2025). Green approach for selective Cu(II) recovery from aqueous solutions: Efficient separation from Ni(II) and Co(II) with organic phase regeneration. Asia-Pacific Journal of Science and Technology, 30(06), APST–30. https://doi.org/10.14456/apst.2025.96
References
Ayyappadas C, Muthuchamy A, Raja Annamalai A, Agrawal DK. An investigation on the effect of sintering mode on various properties of copper-graphene metal matrix composite. Adv Powder Technol. 2017;28:1760-1768.
Marenych O, Kostryzhev A. Strengthening mechanisms in nickel-copper alloys: A review. Metals. 2020;10:11-18.
Duan C, Ma T, Wang J, Zhou Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J Water Process Eng. 2020;37:101339.
Hu H, Li X, Huang P, Zhang Q, Yuan W. Efficient removal of copper from wastewater by using mechanically activated calcium carbonate. J Environ Manage. 2017;203:1–7.
Bediako JK, Choi JW, Song MH, Yun YS. Strategies for recovery of copper and gold as single constituents or an alloy: Selective separation and adsorption-coupled incineration of the bulk metal-loaded adsorbents. Resour Conserv Recycl. 2022;181:106264.
Chang SH, Jampang AOA. Enhanced adsorption selectivity of Au (III) over Cu (II) from acidic chloride solutions by chitosan / palm kernel fatty acid distillate / magnetite nanocomposites. Int J Biol Macromol. 2023;252:126491.
Mehdipoor MA, Moosavirad SM. Effect of holed ferrum electrodes (HFE) on the efficiency of the electrocoagulation process for copper recovery and optimization of parameters, using RSM. Hydrometallurgy. 2020;194:105313.
Liu W, Li W, Liu W, Shen Y, Zhou S, Cui B. A new strategy for extraction of copper cyanide complex ions from cyanide leach solutions by ionic liquids. J Mol Liq. 2023;383:122108.
Chang SH. Micro/nanomotors for metal ion detection and removal from water: A review. Mater Today Sustain. 2022;19:100196.
Zhu G, Wang Y, Huang Q, Zhang R, Chen D, Wang S, Yang X. Emulsion liquid membrane for simultaneous extraction and separation of copper from nickel in ammoniacal solution. Miner Eng. 2022;188:107849.
Rajendaren V, Saufi SM, Zahari MAK, Mohammad AW. Study on stripping phase conditions on the levulinic acid extraction using supported liquid membrane. J Mech Eng Sci. 2019;13:5625–5636.
Wazeer I, Hizaddin HF, Hashim MA, Hadj-Kali MK. An overview about the extraction of heavy metals and other critical pollutants from contaminated water via hydrophobic deep eutectic solvents. J Environ Chem Eng. 2022;10:108574.
Chang SH. Utilization of green organic solvents in solvent extraction and liquid membrane for sustainable wastewater treatment and resource recovery – a review. Environ Sci Pollut Res. 2020;27:32371–32388.
Lee LY, Morad N, Ismail N, Talebi A, Rafatullah M. Optimization for liquid-liquid extraction of Cd(II) over Cu(II) ions from aqueous solutions using ionic liquid aliquat 336 with tributyl phosphate. Int J Mol Sci. 2020;21:1–16.
Qiu Y, Yang L, Huang S, Ji Z, Li Y. The separation and recovery of copper(II), nickel(II), cobalt(II), zinc(II), and cadmium(II) in a sulfate-based solution using a mixture of Versatic 10 acid and Mextral 984H. Chin J Chem Eng. 2017;25:760–767.
Zhu Z, Zhang W, Pranolo Y, Cheng CY. Separation and recovery of copper, nickel, cobalt and zinc in chloride solutions by synergistic solvent extraction. Hydrometallurgy. 2012;127-128:1–7.
Sridhar V, Verma JK. Extraction of copper, nickel and cobalt from the leach liquor of manganese-bearing sea nodules using LIX 984N and ACORGA M5640. Miner Eng. 2011;24:959–962.
Youcef MH, Reffas H, Benabdallah T. Comparative study on extraction of copper(II) cations from highly saline media using 2-((phenylimino)methyl) phenol chelating mono-Schiff base /kerosene as novel extractant system. J Environ Chem Eng. 2021;9:106351.
Chang SH. A comparative study of batch and continuous bulk liquid membranes in the removal and recovery of Cu(II) ions from wastewater. Water Air Soil Pollut. 2018;229:1-22.
Janssen CHC, Macías-Ruvalcaba NA, Aguilar-Martínez M, Kobrak MN. Copper extraction using protic ionic liquids: Evidence of the Hofmeister effect. Sep Purif Technol. 2016;168:275–283.
Martin MI, Garcia-Diaz I., Lopez FA. Properties and perspective of using deep eutectic solvents for hydrometallurgy metal recovery. Miner Eng. 2023;203:108306.
Halim SFA, Chang SH, Morad N. Parametric studies of Cu(II) ion extraction into palm kernel fatty acid distillate as a green organic solvent. J Environ Chem Eng. 2019;7:103488.
Halim SFA, Morad N, Chang SH. Palm kernel fatty acid distillate as a benign organic solvent for Cu(II) extraction: stoichiometry, thermodynamic and structural studies. Chemical Papers. 2023;77:7237-7248.
Halim SFA, Chang SH, Morad N. Extraction of Cu(II) ions from aqueous solutions by free fatty acid-rich oils as green extractants. J Water Process Eng. 2020;33:100997.
Chang SH, Jampang AOA. Green extraction of Gold(III) and Copper(II) from chloride media by palm kernel fatty acid distillate. J Water Process Eng. 2021;43:102298.
Chang R, Goldsby KA, Chemistry, 11th ed., Mc Graw Hill, New York, 2013.
Ebbing DD, Gammon SD, General Chemistry, 9th ed., Charles Hartford, Boston New York, 2007.
Silberberg MS, Principles of General Chemistry, 3rd ed., McGraw Hill, New York, 2013.
Wilson AM, Bailey PJ, Tasker PA, Turkington JR, Grant RA, Love JB, Solvent extraction: the coordination chemistry behind extractive metallurgy. Chem Soc Rev. 2014;43:123–134.
Rydberg GRCJ, Cox M, Musikas C. Solvent extracion principles and practice, second. United States of America: Marcel Dekker Inc; 2004.
Azizitorghabeh A, Rashchi F, Babakhani A. Stoichiometry and structural studies of Fe(III) and Zn(II) solvent extraction using D2EHPA/TBP. Sep Purif Technol. 2016;171:197–205.
Stuart B. Infrared Spectroscopy: Fundamentals and Applications, John Wiley & Sons, Inc, The United Kingdom, 2004.

Published: Nov 4, 2025
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
