Muinat Olanike Kazeem
Department of Microbiology, Faculty of Life Science, University of Ilorin, Kwara State, Nigeria
Emmanuel Boluwatife Akanbi
Department of Microbiology, Faculty of Life Science, University of Ilorin, Kwara State, Nigeria
Gbemisola Elizabeth Ogunleye
Department of Biological Sciences, Faculty of Applied Sciences, KolaDaisi University, Oyo State, Nigeria
Kubrat Abiola Oyinlola
Department of Microbiology, Faculty of Science, University of Ibadan, Oyo State, Nigeria
DOI: https://doi.org/10.14456/apst.2025.56
Keywords: Cellulase Immobilization Iron oxide Nanoparticles Scanning Electron Microscopy Energy Dispersion X-ray Fourier Transform Infrared Spectroscopy Re-usability
Abstract
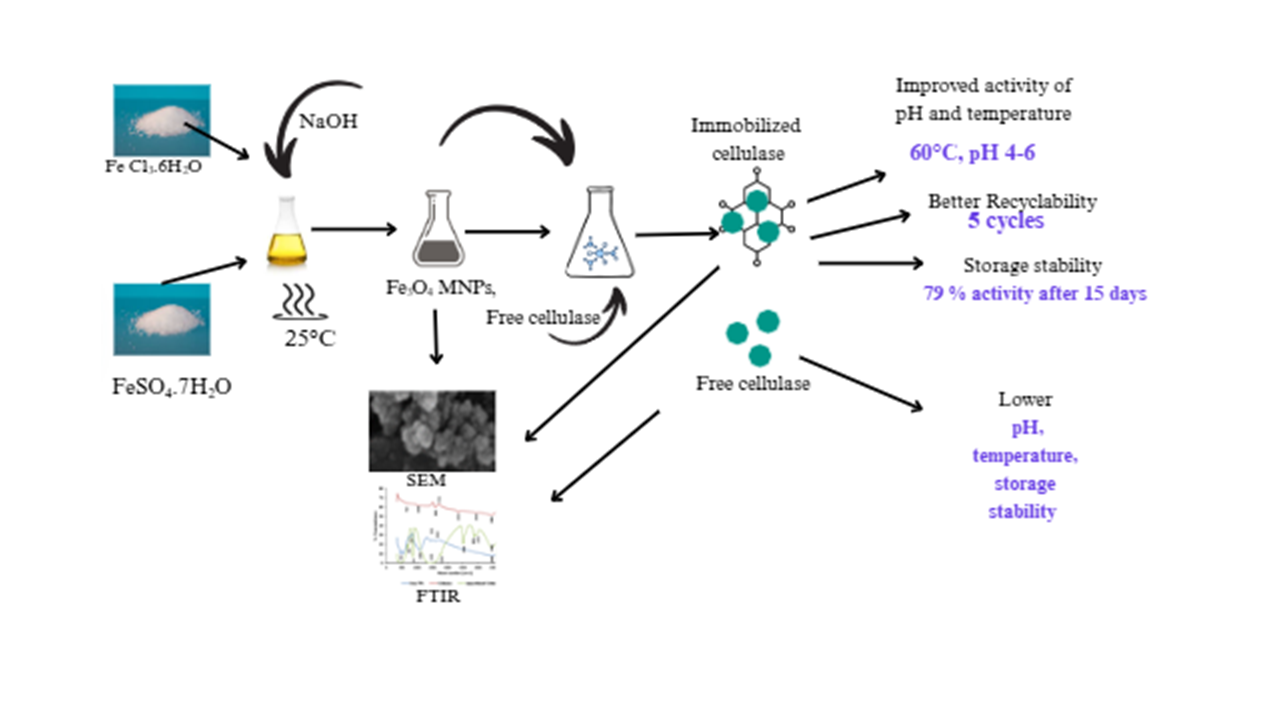
The high sensitivity to pH and temperature, as well as separation difficulties of free cellulase restrict efficient lignocellulose biotransformation. Enzyme immobilization on magnetic nanoparticles offers a new technique for stabilizing enzymes, with easy reuse. The study reports the synthesis of iron oxide magnetic nanoparticles (Fe3O4 MNps) for cellulase immobilization. By co-precipitating Fe2+ and Fe3+ in a 2:1 molar ratio, the Fe3O4 Nps was created. Cellulase was immobilized using glutaraldehyde as a cross linker. UV-Vis spectrophotometry, Fourier transform infrared (FTIR) Spectroscopy, Energy Dispersed X-ray spectroscopy and Scanning Electron Microscopy were studied to determine the surface plasmon resonance (SPR) band, functional group, element, size, shape and binding of the cellulase to the Nps. The impact of pH, temperature, stability and reusability of the immobilized enzyme were investigated. UV-peak at 320 nm indicates small nanoparticle size while FTIR bands at 500-700 cm indicate iron oxide Fe-O bonds vibrations. A characteristic peak at 1441 cm and 1435 cm for nanoparticles and immobilized cellulase indicates uncoordinated carbonate anion. Agglomeration of iron oxide nanoparticles (20 nm) was observed after cellulase immobilization. The optimum temperature of free cellulase shifted from 50 to 60°C after immobilization and the pH was stable at a range of 4 to 7. Better thermal and storage stability were displayed by the immobilized cellulase, which retained 71% activity after the fifth cycle of reuse. Immobilized cellulase-Fe3O4 MNps outperformed free cellulase in terms of pH tolerance, thermal stability, and storage stability. Additionally, because it can be recycled numerous times, it is commercially viable.
How to Cite
Kazeem, M. O. ., Akanbi, E. B. ., Ogunleye, G. E., & Oyinlola, K. A. . (2025). Immobilization and characterization of cellulase on iron oxide nanoparticles for efficient re-usability. Asia-Pacific Journal of Science and Technology, 30(04), APST–30. https://doi.org/10.14456/apst.2025.56
References
Hanefeld U, Gardossi L, Magner E. Understanding enzyme immobilisation. Chem Soc Rev. 2009;38(2):453-468.
Verma ML, Puri M, Barrow CJ. Recent trends in nanomaterials immobilised enzymes for biofuel production. Crit Rev Biotechnol. 2016;36(1):108-119.
Thangaraj B, Solomon PR. Immobilization of Lipases – A Review. Part I: Enzyme immobilization. Chem Bio Eng Rev. 2019;6(5):157-166.
Galvão W, Pinheiro B, Golçalves L, De Mattos M, Fonseca T, Regis T, et al. Novel nanohybrid biocatalyst: application in the kinetic resolution of secondary alcohols. J Mater Sci. 2018;53(20):14121-14137.
Prasad J, Singh AK, Haldar KK, Tomar M, Gupta V, Singh K. CoFe 2 O 4 nanoparticles decorated MoS 2-reduced graphene oxide nanocomposite for improved microwave absorption and shielding performance. RSC Advances. 2019;9(38):21881-21892.
Selvam K, Govarthanan M, Senbagam D, Kamala-Kannan S, Senthilkumar B, Selvankumar T. Activity and stability of bacterial cellulase immobilized on magnetic nanoparticles. Chinese J Catal. 2016;37(11):1891-1898.
Antony VS, Sahithya CS, Durga Sruthi P, Selvarani J, Raji P, Prakash P, et al. Itraconazole coated super paramagnetic iron oxide nanoparticles for antimicrobial studies. Biointerface Res Appl Chem. 2020; 10:6218-6225.
Eivazzadeh-Keihan R, Bahreinizad H, Amiri Z, Aliabadi HAM, Salimi-Bani M, Nakisa A, et al. Functionalized magnetic nanoparticles for the separation and purification of proteins and peptides. Trends Analyt Chem. 2021;141:116-291.
Tang C, He Z, Liu H, Xu Y, Huang H, Yang G, et al. Application of magnetic nanoparticles in nucleic acid detection. J Nanobiotechnol. 2020;18(1):1-19.
Yoneyama T, Kuwahata A, Murayama T, Tonthat L, Yabukami S, Sato Y, et al. Simplified fabrication of magnetic nanoparticles with directly adsorbed antibodies for bacteria detection. IEEE Transactions on Magnetics. 2022;58(8):1-6.
Dudchenko N, Pawar S, Perelshtein I, Fixler D. Magnetite nanoparticles: Synthesis and applications in optics and nanophotonics. Materials. 2022;15(7):26-101.
Darwesh OM, Ali SS, Matter IA, Elsamahy T, Mahmoud YA. Enzymes immobilization onto magnetic nanoparticles to improve industrial and environmental applications. Methods Enzymol: Elsevier. 2020;1: 481-502.
Jiang Y, Guo C, Xia H, Mahmood I, Liu C, Liu H. Magnetic nanoparticles supported ionic liquids for lipase immobilization: Enzyme activity in catalyzing esterification. J Mol Catal B Enzymatic. 2009;58(1-4):103-109.
Ren Y, Rivera JG, He L, Kulkarni H, Lee D-K, Messersmith PB. Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotechnol. 2011; 11:1-8.
Kazeem MO, Shah UKM, Baharuddin AS, AbdulRahman NA. Prospecting Agro-waste Cocktail: Supplementation for Cellulase Production by a newly isolated thermophilic B. licheniformis 2D55. Appl Biochem Biotechnol. 2017;182(4):1318-1340.
Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analyt Chem. 1959;31(3):426-428.
Kaur P, Taggar MS, Kalia A. Characterization of magnetic nanoparticle–immobilized cellulases for enzymatic saccharification of rice straw. Biomass Convers Biorefin. 2021;11:955-969.
Mohamed SA, Al-Harbi MH, Almulaiky YQ, Ibrahim IH, El-Shishtawy RM. Immobilization of horseradish peroxidase on Fe3O4 magnetic nanoparticles. Electron J Biotechnol. 2017;27:84-90.
Hwang S, Umar A, Dar G, Kim S, Badran R. Synthesis and characterization of iron oxide nanoparticles for phenyl hydrazine sensor applications. Sensor Lett. 2014;12(1):97-101.
Jordan J, Kumar CS, Theegala C. Preparation and characterization of cellulase-bound magnetite nanoparticles. J Mol Catal B: Enzymatic. 2011;68(2):139-146.
Kouassi GK, Irudayaraj J, McCarty G. Examination of cholesterol oxidase attachment to magnetic nanoparticles. J Nanobiotechnol. 2005;3:1-9.
Khan MR. Immobilized enzymes: a comprehensive review. Bulletin Nat Res Centre. 2021;45(1):20-27.
Sillu D, Agnihotri S. Cellulase immobilization onto magnetic halloysite nanotubes: enhanced enzyme activity and stability with high cellulose saccharification. ACS Sust Chem Eng. 2019;8(2):900-13.
Tao Q-L, Li Y, Shi Y, Liu R-J, Zhang Y-W, Guo J. Application of molecular imprinted magnetic Fe3O4 SiO2 nanoparticles for selective immobilization of cellulase. J Nanosci Nanotechnol. 2016;16(6):6055-60.
Salem K, Jabalera Y, Puentes-Pardo JD, Vilchez-Garcia J, Sayari A, Hmida-Sayari A, et al. Enzyme storage and recycling: Nanoassemblies of α-amylase and xylanase immobilized on biomimetic magnetic nanoparticles. ACS Sust Chem Eng. 2021;9(11):4054-63.
Dhavale R, Parit S, Sahoo SC, Kollu P, Patil P, et al. α-amylase immobilized on magnetic nanoparticles: reusable robust nano-biocatalyst for starch hydrolysis. Mat Res Express. 2018;5(7):075403.
Landarani-Isfahani A, Taheri-Kafrani A, Amini M, Mirkhani V, Moghadam M, Soozanipour A, et al. Xylanase immobilized on novel multifunctional hyperbranched polyglycerol-grafted magnetic nanoparticles: an efficient and robust biocatalyst. Langmuir. 2015;31(33):9219-9227.
Sohrabi N, Rasouli N, Torkzadeh M. Enhanced stability and catalytic activity of immobilized α-amylase on modified Fe3O4 nanoparticles. Chem Eng J. 2014; 240:426-433.
Jia J, Zhang W, Yang Z, Yang X, Wang N, Yu X. Novel magnetic cross-linked cellulase aggregates with a potential application in lignocellulosic biomass bioconversion. Molecules. 2017;22(2):269-270.
Sánchez-Ramírez J, Martínez-Hernández JL, Segura-Ceniceros P, López G, Saade H, Medina-Morales MA, et al. Cellulases immobilization on chitosan-coated magnetic nanoparticles: application for Agave Atrovirens lignocellulosic biomass hydrolysis. Bioprocess Biosyst Eng. 2017;40(1):9-22.
Muley AB, Mulchandani KH, Singhal RS. Immobilization of enzymes on iron oxide magnetic nanoparticles: Synthesis, characterization, kinetics and thermodynamics. Methods Enzymol. 2020;630:39-79.

Published:
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
