Nisar Ahmad
Center for Biotechnology and Microbiology, University of Swat, Swat 19200, Pakistan
Sidra Mukhtar
Department of Horticulture, Faculty of Crop Production Sciences, The University of Agriculture, Peshawar 25120, Pakistan
Naveed Ahmad
Department of Horticulture, Faculty of Crop Production Sciences, The University of Agriculture, Peshawar 25120, Pakistan
Muhammad Sajid
Department of Horticulture, Faculty of Crop Production Sciences, The University of Agriculture, Peshawar 25120, Pakistan
Irfan Ullah
Department of Horticulture, Faculty of Crop Production Sciences, The University of Agriculture, Peshawar 25120, Pakistan
Nadia Samad
Department of Horticulture, Faculty of Crop Production Sciences, The University of Agriculture, Peshawar 25120, Pakistan
Muhammad Numan
Department of Agricultural Chemistry, Faculty of Nutrition Sciences, The University of Agriculture, Peshawar 25120, Pakistan
Zafar Iqbal
Department of Agricultural Chemistry, Faculty of Nutrition Sciences, The University of Agriculture, Peshawar 25120, Pakistan
Hina Fazal
Pakistan Council of Scientific and Industrial Research (PCSIR) Laboratories Complex, Peshawar 25120, Pakistan
Mohammad Ali
Center for Biotechnology and Microbiology, University of Swat, Swat 19200, Pakistan
Hassan Sher
Center for Plant Sciences and Biodiversity, University of Swat, Swat 19200 Pakistan
DOI: https://doi.org/10.14456/apst.2025.35
Keywords: Stevia rebaudiana Morphogenesis Gelling agents Isubgol Sago Semolina Corn starch BAP
Abstract
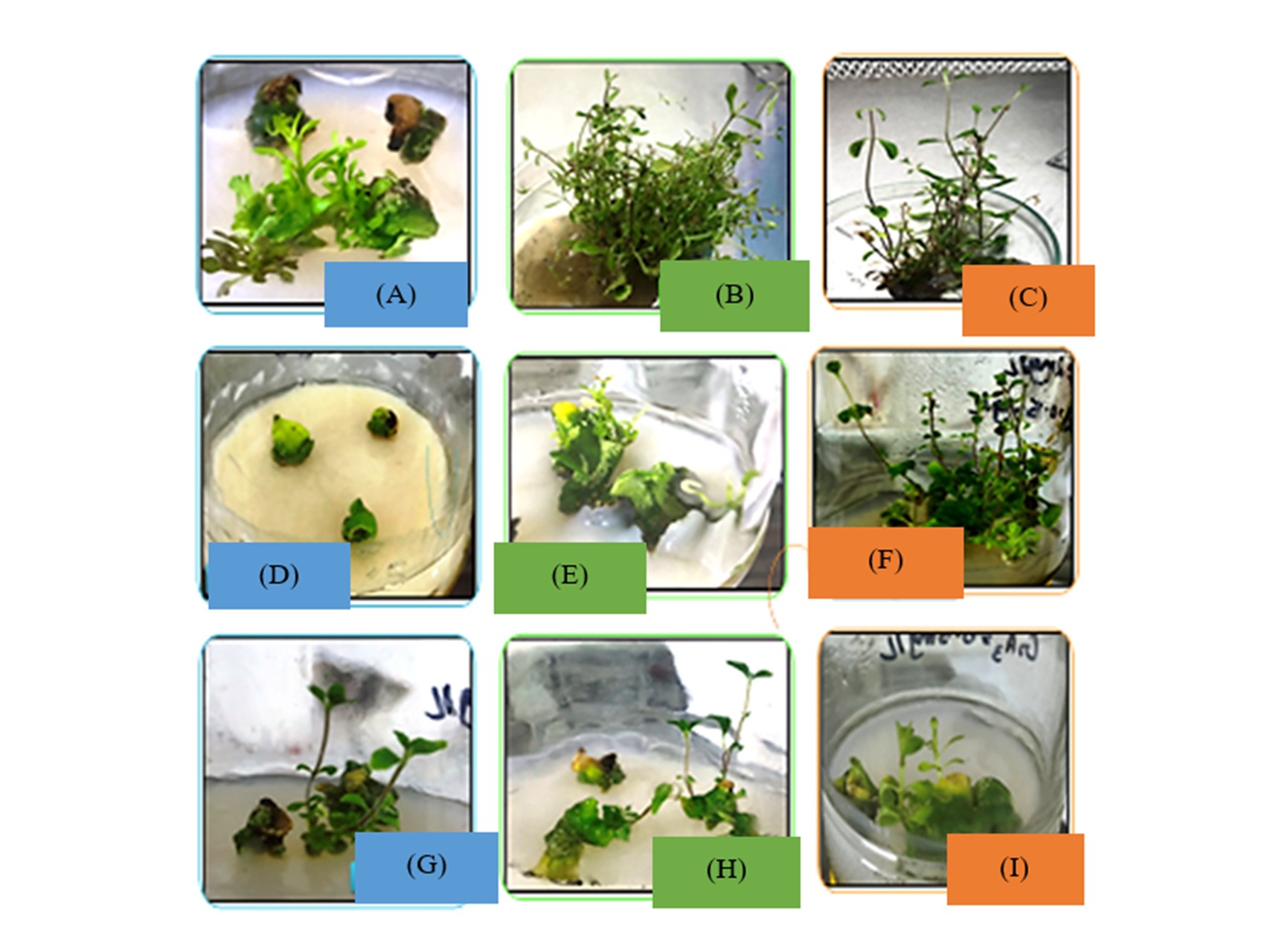
Plant cell culture is an advance technology for large scale production of medicinal plants of commercial importance but high cost of the media is one of the major issues for economical growers. One of the most expensive agent of culture media is agar and therefore, the main objective of this study was to select best agar alternative among isubgol, semolina, laundry starch, sago, and corn starch. These alternatives were tested during the morphogenesis of Stevia rebaudiana (Bert.). S. rebaudiana (Bert.) is one the famous sweet herb that produce sweet diterpenes that could be used as sugar alternative for diabetic patients due to its non-toxic nature. Here, the synergistic combinations of isubgol and Agar+isubgol augmented Murashige and Skoog (MS) media supplemented with 2.0 mg/L benzyl aminopurine (BAP) and 0.5 mg/L gibberellic acid (GA3) was found to be effective for shoot induction (100%) and shoot morphogenesis than control (MS media without plant growth regulators). The MS media supplemented with 0.5 mg/L naphthalene acetic acid (NAA) along with corn starch (90 g/L) is the best combination for rapid root initiation, however, corn starch/sago alone or the synergistic combination of with agar or sago significantly improved root organogenesis (100%) in medicinally important Stevia rebaudiana (Bert.). However, sago encourage mean shoot and root length and reduced 90% cost than agar. Semolina was the cheapest agar substitute by reducing the cost up to 97.75% and could be scale up for higher production of plantlets of commercial importance.
How to Cite
Ahmad, N., Mukhtar, S., Ahmad, N., Sajid, M., Ullah, I., Samad, N., Numan, M., Iqbal, Z., Fazal, H., Ali, M., & Sher, H. (2025). Large scale production of in vitro plantlets of Stevia rebaudiana (Bertoni) using low-cost gelling agents as alternative to commonly used agar . Asia-Pacific Journal of Science and Technology, 30(03), APST–30. https://doi.org/10.14456/apst.2025.35
References
Ahmad N, Fazal H, Zamir R, Khalil SA, Abbasi BH. Callogenesis and Shoot organogenesis from flowers of Stevia rebaudiana (Bert.). Sugar tech. 2011;13:174-177.
Aman N, Hadi F, Khalil SA, Zamir R, Ahmad N. Efficient regeneration for enhanced steviol glycosides production in Stevia rebaudiana (Bertoni). C R Biol. 2013;336:486–492.
Khalil SA, Zamir R, Ahmad N. Selection of suitable propagation method for consistent plantlets production in Stevia rebaudiana (Bertoni). Saudi J Biol Sci. 2014;24(6):1743-1751.
Khalil, SA, Kamal N, Sajid M, Ahmad N, Zamir R, Ahmad N, Ali S. Synergism of polyamines and plant growth regulators enhanced morphogenesis, stevioside content and production of commercially important natural antioxidants in Stevia rebaudiana (Bert.) In Vitro Cell & Dev Biology – Plant. 2016; DOI 10.1007/s11627-016-9749-6.
Ahmad N, Rab A, Sajid M, Ahmad N, Fazal H, Ali M, Egertsdotter U. Sucrose-dependent production of biomass and low-caloric steviol glycosides in adventitious root cultures of Stevia rebaudiana (Bert.). Ind Crop Prod. 2021;164;2021:113382.
Ahmad A, Ali H, Khan H, Begam A, Khan S, Ali SS, Ahmad N, Fazal H, Ali M, Hano C, Ahmad N, Abbasi BH. Effect of Gibberellic Acid on Production of Biomass, Polyphenolics and Steviol Glycosides in Adventitious Root Cultures of Stevia rebaudiana (Bert.). Plants. 2020;9(4):420; doi:10.3390/plants9040420.
Ahmad N, Rab A, Ahmad N, Fazal H. Differential pH-Induced Biosynthesis of Steviol Glycosides and Biochemical Parameters in Submerge Root Cultures of Stevia rebaudiana (Bert.). Sugar Tech. 2018: https://doi.org/10.1007/s12355-018-0589-z.
Gantait S, Das A, Banerjee J. Geographical distribution, botanical description and self-incompatibility mechanism of genus Stevia – a review. Sugar Tech, 2018;20(1):1-10.
Gantait S, Das A, Mandal N. Stevia: a comprehensive review on ethnopharmacological properties and in vitro regeneration. Sugar Tech, 2015;17(2):95–106.
Ahmad N, Khan P, Khan A, Usman M, Fazal H, Ali M, Abbasi BH, Hano C. Elicitation of submerged Adventitious Root Cultures of Stevia rebaudiana with Cuscuta reflexa for Production of Biomass and Secondary Metabolites. Molecules, 2022;27(1):14.
Jihad S, El-Khayat GH, Manthey FA, Fuller MP, Brennan CS. Durum wheat quality the relationship of kernel physicochemical composition to semolina quality and end product utilization. Int J Food Sci Tech. 2006;2:47-55.
Karim, A. A., A. Pei‐Lang Tie, D. M. A. Manan, and I. S. M. Zaidul. Starch from the sago (Metroxylon sagu) palm tree—properties, prospects, and challenges as a new industrial source for food and other uses. Compr Rev Food Sci Food Saf. 2008;7:3: 215-228
Arthur GD, Stirk WA, Stadan JV. Screening of aqueous extracts from gelling agents (Agar and Gelrite) for root-stimulating activity. South Afr J Bot. 2004;4:595-601.
Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant. 1962;15:473–497.
Jain R, Babbar SB. Guar gum and isubgol as cost-effective alternative gelling agents for in vitro multiplication of an orchid, Dendrobium chrysotoxum. Curr Sci. 2005;88:292–295.
Norhayati D, Taha RM, Noor NNM, Alimon H. Potential of alternative gelling agents in media for the in vitro micro-propagation of Celosia sp. Int J Bot. 2011;2:183-188.
Saraswathi MS, Uma S, Kannan G, Selvasumathi M, Mustaffa MM, Backiyarani S. Cost-effective tissue culture media for large-scale propagation of three commercial banana (Musa spp.) varieties. The J Hort Sci Bio. 2016;1:23-29.
Ullah MA, Uddin MI, Puteh AB, Haque MS, Islam MS. Alternative gelling agents for in vitro propagation of orchid (Dendrobium sonia). The J Animal Plant Sci. 2015;3:792-797.
Sharifil A, Moshtaghi N, Bagheri A. Agar alternatives for micropropagation of African violet (Saintpaulia ionantha). Afr J Biotech. 2010;9:9199-9203.
Shailaja PV, Patil SS. Potential substitutes for agar in tissue culture media. J Orn Hort. 2004;7:247-250.
Rishsi KT, Agrawal A, Mahalakshmi C, Hussain Z, Tyagi H. Low-cost media for in vitro conservation of turmeric (Curcuma longa L.). Physiol Plant. 2007;59:260-266.
Bhattacharya P, Dey S, Bhattacharyya BC. Use of low-cost gelling agents and support matrices for industrial scale plant tissue culture. Plant Cell Tiss Org Cult. 1994;37:15-23.
Prabhuling G, Mastiholi AB, KerutagI MG. Low cost gelling agents for micro-propagation of banana (Musa acuminate) cv. ‘GRANDE NAINE’. Int J Plant Sci. 2014;1:46-51.
Prabhakara HL. Studies on in vitro propagation of Anthurium andreanum Lind. M.Sc. (Ag.) Thesis, Uni of Agri Sci, Dharwad, karnataka (INDIA).1999.
Naik PS, Sarkar D. Sago, an alternative cheap gelling agent for potato in vitro culture. Biol Plant. 2001;2:293-296.
Anagnostakis SL. Haploid plants from anthers of tobacco enhancement with charcoal. Plant. 1974;15:281–283.
Onwueme IC. The Tropical Crops, Yams, Cassava, Sweet potatoes and Cocoyam. University of Ife; Ile-Ife, Nigeria. 1982;145.
Ozel CA, Khawar KM, Arslan O. A comparison of the gelling of isubgol, agar and gelrite on in vitro shoot regeneration and rooting of variety Samsun of tobacco (Nicotiana tabacum L.). Sci Hort. 2008;117:174-181.
George EF, Hall MA, De Klerk GJ. The components of plant tissue culture media II: Organic additions, osmotic and pH effects, and support systems. In: George EF, Hall MA, De Klerk GJ, editors. Plant propagation by tissue culture. 3rd ed. Dordrecht: Springer; 2008. p. 115–173.
Ivanova M, Staden JV. Influence of gelling agent and cytokinins on the control of hyperhydricity in Aloe polyphylla. Plant Cell Tiss Org Cult. 2011;1:13-21.
Takahito I, Koda T, Asai I, Hatanaka A, Sekiya J. Effects of Gelling Agents on in Vitro Culture of Plant Tissues. Agri Bio Chem. 2014;9:2397-2399.
Kuria P, Demo P, Nyende A, Kahangi EM. Cassava starch as an alternative cheap gelling agent for the in vitro micro-propagation of potato (Solanum tuberosum L.). Afr J Biotech. 2008;3:301-307.
Soejarto DD, Kinghorn AD, Farnsworth NR. Potential sweetening agents of plant origin. III. Organoleptic evaluation of Stevia leaf herbarium samples for sweetness. J Nat Pro. 1982;5:590-599.
Perata P, Matsukura C, Vernieri P, Yamaguchi J. Sugar repression of a gibberelline dependent signaling pathway in barley embryos. The Plant Cell. 1997;9:2197-2208.
Rahman MA, Blake J. The effect of medium composition and culture condition on in vitro rooting and ex vitro establishment of jack fruit (Artocarpas hetrophyllus Lam.). Plant Cell Tiss Org Cult. 1988;13:189-200.
Buah JN. Suitability of cassava starch as a gelling agent for the in vitro culture of banana plantlets. American J Food Tech. 2014;7:340-349.
Richard M, Mneney E, Misangu R, Maerere A. Characteristics of botanical starches as potential substitutes of agar in tissue culture media. Acad. J. 2015;8:702-713.
Laha S, Subrahmanyeswari T, Verma SK, Kamble SN, Bhattacharyya S, Gantait S. Biogenic synthesis, characterization and application of silver nanoparticles as biostimulator for growth and rebaudioside-A production in genetically stable stevia (Stevia rebaudiana Bert.) under in vitro conditions. Ind Crop Prod. 2023;197: 116520.
Subrahmanyeswari T, Laha S, Kamble SN, Singh S, Bhattacharyya S, Gantait S. Alginate-encapsulation of shoot tips and their regeneration for enhanced mass propagation and germplasm exchange of genetically stable Stevia rebaudiana Bert. Sugar Tech, 2023a;25:542-551.
Subrahmanyeswari T, Gantait S, Kamble SN, Singh S, Bhattacharyya S meta-Topolin-induced regeneration and ameliorated rebaudioside-A production in genetically uniform candy-leaf plantlets (Stevia rebaudiana Bert.). South Afr J Bot. 2023b;159:405–418.

Published:
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
