Janus B Pansacala
Department of Chemistry, Western Mindanao State University, Zamboanga City, Philippines
Joshua Andrew P Nillama
Department of Chemistry, Mindanao State University-Iligan Institute of Technology, Iligan City, Philippines
Evelyn C Creencia
Department of Chemistry, Mindanao State University-Iligan Institute of Technology, Iligan City, Philippines
Keywords: benzimidazoles green chemistry P2O5/SiO2 room-temperature synthesis
Abstract
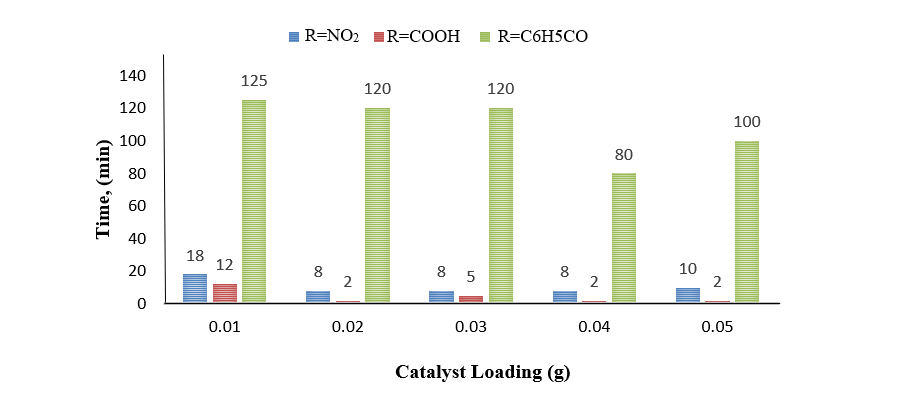
Twelve (12) 2-Aryl-1H-benzimidazole derivatives were synthesized from a series of 4-o-phenylenediamines [R: -NO2, -COOCH2CH3, and -COAr] and 4-aryl aldehydes [R: -H, -N(CH2CH3)2, -CH(CH3)2, -CH(OCH2CH3)2] utilizing a simple, cheap, and heterogeneous P2O5/SiO2 catalytic system at room temperature and solvent-free conditions. Moreover, the bioactivity of the prepared benzimidazoles, evaluated via brine shrimp lethality assay (BSLA), showed that almost all benzimidazole products were cytotoxic, except for 5-nitro-2-phenyl-1H-benzo[d]imidazole (5a). Highest activity was observed for 2-(4-isoproylphenyl)-5-nitro-1H-benzo[d]imidazole (5b) with acute lethal concentration (LC50) of 43.42 ppm, whereas the least active N, N-diethyl-4-(5-nitro-1H-benzo[d]imidazol-2-yl) aniline (5c), had chronic LC50 value of 283.39 ppm. Qualitative structure – BSLA activity correlation showed that balanced electron-donating capacity of R2 coupled with electron-withdrawing effect of R1 led to enhanced toxicity of 2-Aryl-1H-benzimidazoles.
How to Cite
Pansacala, J. B. ., Nillama, J. A. P. ., & Creencia, E. C. . (2025). P2O5/SiO2-Catalyzed solvent-free room-temperature synthesis of 2-aryl-benzimidazoles and their toxicity against Artemia salina. Asia-Pacific Journal of Science and Technology, 30(06), APST–30. https://doi.org/10.14456/apst.2025.90
References
Panda SS, Malik R, Jain CS. Synthetic approaches to 2-arylbenzimidazoles: A review. Curr Org Chem. 2012;16:1905–1919.
Gajanan G., Shital S, Vipul T, Babar V, Dnyaneshwar J, Vaibhav D, Vaibhav C. A review on benzimidazole and its biological activities. J Pharm Chem Drug Form. 2021;3:23-32.
Dahaner M, De Ruyck H, Crooks SRH, Dowling G, O’Keeffe M. New methodology for the determination of benzimidazoles in biological matrices. J Chromatography B Anal Technol Biomed Life Sci. 2007;845:1–37.
Enumula S, Pangal A, Gazge M, Shaikh JA, Ahmed K. Diverse pharmacological aspects of benzimidazole derivatives: A review. Res J Chem Sci. 2014; 4:78–88.
Rathod CP, Rajurkar RM, Thonte SS., Benzimidazole synthesis and biological evaluation: a review. Indo Am J Pharm Res. 2013;3:2323–2329.
Lai MY, Chen CH, Huang WS, Lin JT, Ke TH, Tsai M H, Wu CC. Benzimidazole/amine-based compounds capable of ambipolar transport for application in single -layer blue-emitting OLED’s and as hosts for phosphorescent emitters. Angew Chemie Int Ed. 2012; 47:581–585.
Muhammad S, Xu H, Janjua MRSA, Su Z, Nadeem M., Quantum chemical study of benzimidazole derivatives to tune the second-order nonlinear optical molecular switching by proton abstraction. Phys Chem Chem Phys. 2012;12:4791–4799.
Shen CH, Hsu SLC, Bulycheva E, Belomoina N. High temperature proton exchange membranes based on poly (arylene ether) s with benzimidazole side groups for fuel cells. J Mater Chem. 2012;12:19269–19275.
Ibrahim MA. Synthetic utilities of o-phenylenediamines: Synthetic approaches for benzimidazoles, quinoxalines and benzo-1,5diazepines. Heterocycles. 2011;83:2689–2730.
Panda SS, Jain SC. Synthesis of 2-arylbenzimidazoles in water. Synth Commun. 2011;41:729–735.
Gawande MB, Bonifácio VDB, Luque R, Branco PS, Varma RS., Benign by design: Catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem Soc Rev. 2013;42:5522–5551.
McLaughlin DJL, Rogers LL, Anderson JE. The use of biological assays to evaluate botanicals. Drug Inf J. 1998;32(2):513–524.
Eshghi H, Rafei M, Karimi M. P2O5/SiO2 As An efficient reagent for esterification of phenols in dry media. Synth Commun. 2011;31:771–774.
Hasaninejad A, Zare A, Sharghi H, Shekouhy M., P2O5/SiO2 An efficient, green and heterogeneous catalytic system for the solvent-free synthesis of N-sulfonyl imines. ARKIVOC. 2008: (11):64-74
Ngutaa J, Mbariaa W, Gakuyab D, Gathumbic P, Biological screening of kenyan medicinal plants using artemia salina L. (Artemiidae). Pharmacol Online. 2011;2:458–478.
Randhawa MA., Calculation of LD50 values from the method of miller and tainter. J Ayub Med Coll Abbottabad. 2009; 21:184–185
Raj J, Chandra M, Dogra TD, Pahuja M, Raina A. Determination of median lethal dose of combination of endosulfan and cypermethrin in wistar rat. Toxicol Int. 2013;20:1–5.
Kobayashi K, Pillai SK. A handbook of applied statistics in pharmacology. 1st edition. Boca Raton, Florida: CRC Press. 2013.
Salem H, Katz SA (Eds). Inhalation Toxicology. 3rd edition. Boca Raton, Florida: CRC Press, 2015.
Eshghi H, Hassankhani A. phosphorus pentoxide supported on silica gel and alumina (P2O5/SiO2, P2O5/Al2O3) as useful catalysts in organic synthesis. J Iran Chem Soc. 2012;9:467–482.
Momeni AR, Bagheri S. Synthesis of 2-substitued benzimidazoles using P2O5-SiO2. Iran J Cat. 2012;2(1):31–35.
Singhal S, Khanna P, Panda SS, Khanna L. Recent trends in the synthesis of benzimidazoles from o-phenylenediamine via nanoparticles and green strategies using transition metal catalysts. J Heterocycl Chem. 2019;56(10):2702–2729.

Published: Nov 3, 2025
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
