Said Ali Akbar
Department of Aquaculture, Faculty of Marine and Fisheries, Universitas Syiah Kuala, Banda Aceh, 23111, Indonesia
Muhammad Hasan
Department of Chemistry Education, Faculty of Teacher Training and Education, Universitas Syiah Kuala. Banda Aceh 23111, Aceh, Indonesia
Sari Afriani
Department of Fisheries Product Industry Technology, Faculty of Marine and Fisheries, Universitas Syiah Kuala, Banda Aceh, 23111, Indonesia
Cut Nuzlia
Department of Aquaculture, Faculty of Marine and Fisheries, Universitas Syiah Kuala, Banda Aceh, 23111, Indonesia
DOI: https://doi.org/10.14456/apst.2025.41
Keywords: Antibacterial Antioxidant activity H. durvillei Phytochemical profiling Macroalgae
Abstract
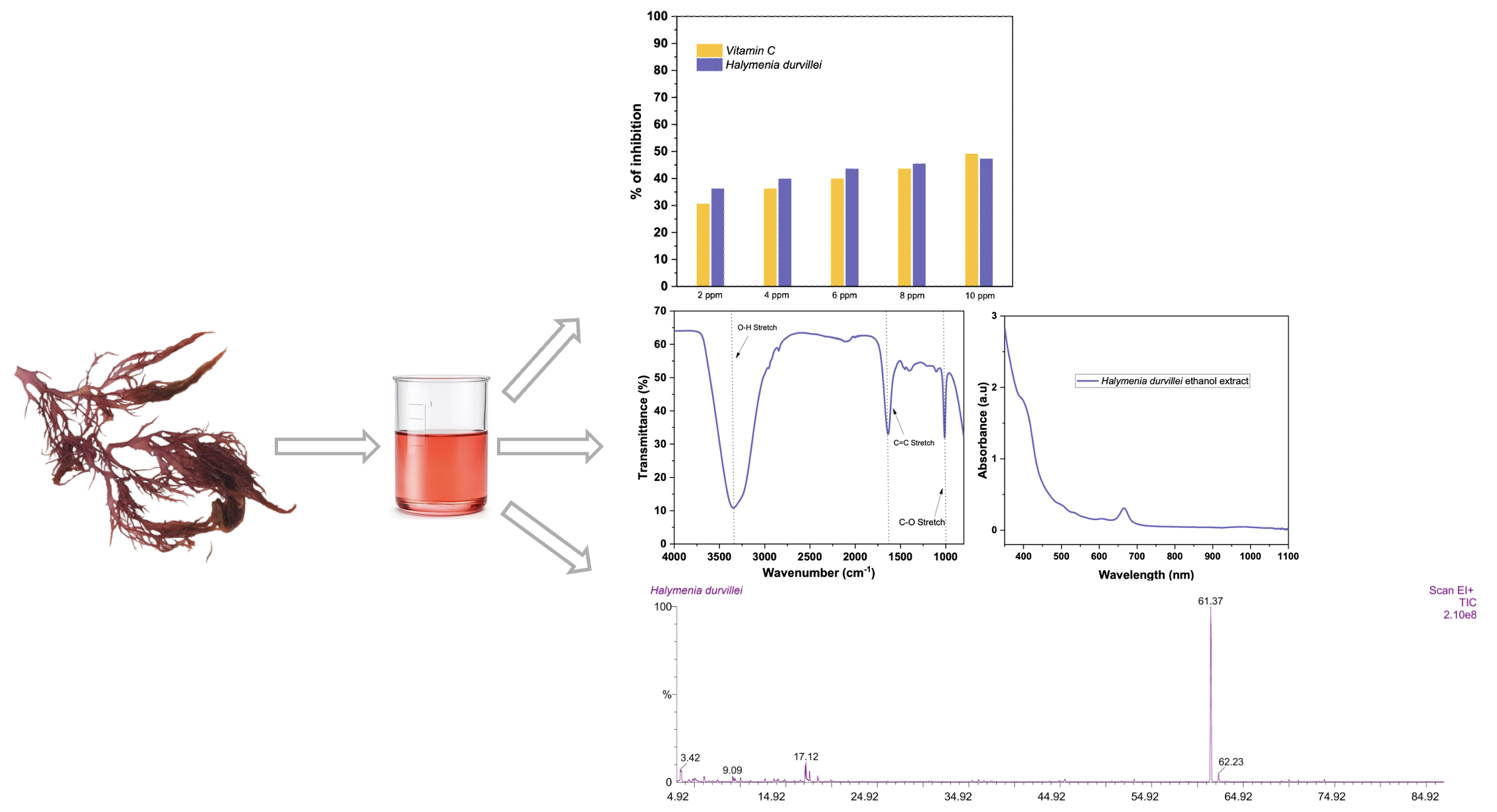
The investigation into seaweed species for the extraction of bioactive compounds has gained significant importance due to its wide-ranging potential applications. This study aimed to assess the metabolite profiles and phytochemical content of Halymenia durvillei ethanol extract along the coastline of Banda Aceh, Indonesia. The methodology proposed in this research encompassed three main steps: analysis of phytochemical composition, evaluation of antioxidant scavenging activity, and in vivo metabolite profiling utilizing gas chromatography-mass spectrometry (GC-MS), Fourier-transform infrared (FTIR) spectroscopy, and the 2,2-diphenylpicrylhydrazyl (DPPH) assay. The utilization of DPPH for assessing antioxidant potential revealed significant scavenging activity, with inhibition values reaching 47.22%. GC-MS analysis demonstrated its efficacy in swiftly and accurately monitoring both individual components and overall chemical composition of H. durvillei ethanol extract. The study identified 26 phytochemical compounds within H. durvillei. Subsequently, the ethanol extract underwent FTIR spectroscopic analysis, revealing prominent peaks at 3352 cm−1, 1654 cm−1, and 1019 cm−1, indicating the presence of alkanes, alkenes, and hydroxyl groups, with other connections ranging from weak to medium. Based on the GC-M S results and literature studies, there are numerous bioactive compounds contained in H. durvillei ethanol extract that have potential as antibacterials in humans and animals, including fish. Consequently, H. durvillei exhibits promise as a source of antibacterial agents, particularly in the context of aquaculture for fish.
How to Cite
Akbar, S. A., Hasan, M. ., Afriani, S. ., & Nuzlia, C. . (2025). Phytochemical Profiling of H. durvillei Ethanol Extract and the Potential Activity as Antibacterial Agents on Fish. Asia-Pacific Journal of Science and Technology, 30(03), APST–30. https://doi.org/10.14456/apst.2025.41
References
Moreira A, Cruz S, Marques R, Cartaxana P. The underexplored potential of green macroalgae in aquaculture. Rev Aquac. 2022;14(1): 5-26.
Filote C, Santos SCR, Popa VI, Botelho CMS, Volf I. Biorefinery of marine macroalgae into high-tech bioproducts: a review. Environ Chem Lett. 2021;19: 969-1000.
Schmitt RJ, Holbrook SJ, Brooks AJ, Adam TC. Evaluating the precariousness of coral recovery when coral and macroalgae are alternative basins of attraction. Limnol Oceanogr. 2022;67: S285-S297.
Akbar SA, Hasan M. Evaluation of Bioactive Composition and phytochemical profile of macroalgae Gracilaria edulis and Acanthophora spicifera from the Banda Aceh Coast, Indonesia Sci Technol Asia. 2024;29:194-207.
Akbar SA, Mustari A. Food packaging based on biodegradable polymers from seaweeds: a systematic review. In BIO Web of Conferences 2024;87:01005.
Ismail MM, Alotaibi BS, El-Sheekh MM. Therapeutic uses of red macroalgae. Molecules. 2020;25(19): 4411.
Fatima R, Priya M, Indurthi L, Radhakrishnan V, Sudhakaran R. Biosynthesis of silver nanoparticles using red algae Portieria hornemannii and its antibacterial activity against fish pathogens. Microb Pathog. 2020;138: 103780.
Hamouda RA, Abd El-Mongy M, Eid KF. Comparative study between two red algae for biosynthesis silver nanoparticles capping by SDS: Insights of characterization and antibacterial activity. Microb Pathog. 2019;129: 224-232.
Kasmiati K, Nurunnisa AT, Amran A, Resya MI, Rahmi MH. Antibacterial activity and toxicity of Halymenia durvillei red seaweed from Kayangan island, South Sulawesi, Indonesia. Fish Aquat Sci. 2022;25(8): 417.
Padayao MH, Padayao FR, Patalinghug JM, Raña GS, Yee J, Geraldino PJ, Quilantang N. Antimicrobial and quorum sensing inhibitory activity of epiphytic bacteria isolated from the red alga Halymenia durvillei. Access Microbiol. 2023;5(12):000563.
Patle TK, Shrivas K, Kurrey R, Upadhyay S, Jangde R, Chauhan R. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV–vis and FTIR spectroscopy. Spectrochim. Acta A Mol Biomol Spectrosc. 2020;242: 118717.
Khalid S, Arshad M, Mahmood S, Siddique F, Roobab U, Ranjha, MMAN, Lorenzo JM. Extraction and quantification of Moringa oleifera leaf powder extracts by HPLC and FTIR. Food Anal Methods. 2023;16(4): 787-797.
Parsa M, Nourani M, Baghdadi M, Hosseinzadeh M, Pejman M. Biochars derived from marine macroalgae as a mesoporous by-product of hydrothermal liquefaction process: characterization and application in wastewater treatment. J Water Process Eng. 2019;32: 100942.
Kim DH, Park MH, Choi YJ, Chung KW, Park CH, Jang EJ, An HJ, Yu BP, Chung HY. Molecular study of dietary heptadecane for the anti-inflammatory modulation of NF-kB in the aged kidney. PLoS One. 2013;8(3): e59316.
Klimjit A, Praiboon J, Tiengrim S, Chirapart A, Thamlikitkul V. Phytochemical composition and antibacterial activity of brown seaweed, Padina australis against human pathogenic bacteria. J Fish Environ. 2021;45(1): 8-22.
Shen N, Wang T, Gan Q, Liu S, Wan L, Jin B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022;383: 132531.
Arguelles E. Bioactive properties of Halymenia durvillei Bory 1828 for pharmaceutical application: antioxidant, antidiabetic, antiwrinkling and skin-whitening activities. Yuzuncu Yıl University J Agric Sci. 2022;32(1): 57-68.
Akbar SA, Lestari AN, Fazli RR, Gunawan G. Harnessing macroalgae for heavy metal phytoremediation: a sustainable approach to aquatic pollution control. BIO Web Conf. 2025;156: 02013.
Akbar SA, Hasan M, Afriani S, Nuzlia C. Evaluation of phytochemical composition and metabolite profiling of macroalgae Caulerpa taxifolia and C. peltata from the Banda Aceh coast, Indonesia. Biodiversitas. 2023;24(10):5283-5292.
Vedhagiri K, Manilal A, Valliyammai T, Shanmughapriya S, Sujith S, Selvin J, Natarajaseenivasan K. Antimicrobial potential of a marine seaweed Asparagopsis taxiformis against Leptospira javanica isolates of rodent reservoirs. Ann Microbiol. 2009;59: 431-437.
Ferdosi MF, Khan IH, Javaid A, Hafiz MS, Butt I, Munir A. GC-MS analysis and bioactive components of flowers of Bergenia ciliata, a weed of rock crevices in Pakistan. Pak J Weed Sci Res. 2021;27(4): 527.
Garba S, Shoge M, Salihu L. Antimicrobial Activity Of N-Octadecanal Isolated From The Seeds And Pods Of Acacia nilotica Linn. Bima J Sci Technol. 2016;1(1): 14-21.
Vaiyapuri M, Joseph TC, Rao BM, Lalitha KV, Prasad MM. Methicillin-resistant Staphylococcus aureus in seafood: prevalence, laboratory detection, clonal nature, and control in seafood chain. J Food Sci. 2019;84: 3341–3351.
Hennekinne J-A, De Buyser M-L, Dragacci S. Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiol Rev. 2012;36: 815–836.
Kumar V, Prasher IB. Antimicrobial potential of endophytic fungi isolated from Dillenia indica L. and identification of bioactive molecules produced by Fomitopsis meliae (Undrew.) Murril Nat Prod Res. 2022;36(23): 6064-6068.
Nasr Z, El-shershaby H, Sallam K, Abed N, Abd-El ghany I, Sidkey N. Evaluation of Antimicrobial Potential of Tetradecane Extracted from Pediococcus acidilactici DSM: 20284 – CM Isolated from Curd Milk. Egypt J Chem. 2022;65(3): 705-713.
Rasyid A. Analysis of secondary metabolites, antibacterial activity and compound composition in the sea cucumber Bohadschia sp. extract. J Ilmu Teknol Kelaut Trop., 2016;8(2): 645-653.
Keskın D, Ceyhan N, Uğur A, beys AD. Antimicrobial activity and chemical constitutions of West Anatolian olive (Olea europaea L.) leaves. J Food Agric Environ. 2012;10(2): 99-102.
Getahun T, Sharma V, Gupta N. Chemical composition, antibacterial and antioxidant activities of oils obtained by different extraction methods from Lepidium sativum L. seeds. Ind Crops Prod. 2020;156: 112876.
Elbalola AA, Abbas ZK. Phytochemical Diversity, Classification and antibacterial activity of some medicinal plant species from Tabuk (Saudi Arabia). Chem Biodivers. 2023;20(7): e202300545.
Zaki NH, Ali AM, AL-Rubaiee GH, Alhammer AH. Anti-bacterial and Anti-tumoral Activities of Spirulina Platensis extracellular extract. Malaysian. J Med Health Sci. 2022;18: 11-16.
Borburema HDDS, Lima RPD, Miranda GECD. Effects of ocean warming, eutrophication and salinity variations on the growth of habitat-forming macroalgae in estuarine environments. Acta Bot Bras. 2021;34: 662-672.
Olli K, Tamminen T, Ptacnik R. Predictable shifts in diversity and ecosystem function in phytoplankton communities along coastal salinity continua. Limnol Oceanogr. 2023;8(1): 173-180.
Akbar SA, Khairunnisa K. Seaweed-based biosorbent for the removal of organic and inorganic contaminants from water: a systematic review. In BIO Web of Conferences. 2024;87: 02011.
Farahdiba AU, Hidayah EN, Asmar GA, Myint YW. Growth and removal of nitrogen and phosphorus by a macroalgae Cladophora glomerata under different nitrate concentrations. Nat Environ Pollut Technol. 2020;19(2): 809-813.

Published:
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
