Pongsak Noparat
Program in Natural Resources and Environment, Faculty of Science and Technology, Suratthani Rajabhat University, Suratthani, Thailand
Jiravut Seengenyoung
ASEAN Biological Engineering Society, Songkhla, Thailand
DOI: https://doi.org/10.14456/apst.2025.5
Keywords: biofuel oil palm trunk hydrolysate Clostridium beijerinckii renewable energy
Abstract
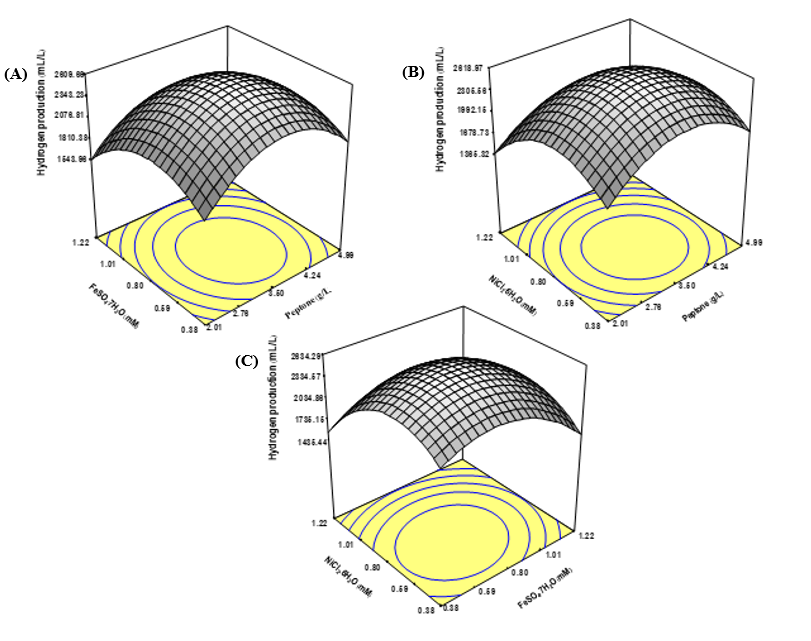
The study aimed to advance biohydrogen production by leveraging oil palm trunk (OPT) hydrolysate as a sustainable feedstock for Clostridium beijerinckii PS-3. Through a controlled hydrolysis process, OPT was treated with H₂SO₄ concentrations from 0.05% to 3.0% (v/v) over reaction times of 5 to 20 minutes at 121ºC. The optimal hydrolysis condition, determined to be 0.1% H₂SO₄ for 15 minutes, produced a hydrolysate rich in fermentable sugars, with a total concentration of 24.02 g/L—comprising 17.04 g/L xylose, 5.11 g/L glucose, 1.87 g/L arabinose, and 2.59 g/L acetic acid. To maximize hydrogen yield, response surface methodology (RSM) and central composite design (CCD) were employed, identifying peptone, iron, and nickel as critical elements influencing production efficiency. Optimal concentrations of 3.50 g/L peptone, 0.80 mM iron, and 0.80 mM nickel were predicted to yield a maximum of 2685 mL/L hydrogen. Experimental validation under refined conditions of 3.25 g/L peptone, 0.63 mM Fe²⁺,and 0.74 mM Ni²⁺,at an initial pH of 6.3 and 30ºC, surpassed predictions, achieving 2832 mL/L cumulative hydrogen and a yield of 236 mL H₂/g total sugar—an 11% increase over conventional media. Triplicate trials confirmed the consistency and robustness of OPT hydrolysate as a substrate for fermentative hydrogen production by C. beijerinckii PS-3. The substantial yield improvement illustrates the potential of OPT hydrolysate as a viable, low-cost resource for biohydrogen, emphasizing the importance of tailored hydrolysis and medium optimization in enhancing bioenergy production processes. Such advancements affirm the role of agricultural waste valorization in achieving economically feasible and environmentally sustainable biofuel solutions.
How to Cite
Noparat, P., & Seengenyoung, J. (2025). Sustainable hydrogen production from oil palm trunk biomass: Optimization of hydrolysis and fermentation conditions with Clostridium beijerinckii PS-3. Asia-Pacific Journal of Science and Technology, 30(01), APST–30. https://doi.org/10.14456/apst.2025.5
References
Sinbuathong N, Sillapacharoenkul B. Dark fermentation of starch factory wastewater with acid- and base-treated mixed microorganisms for biohydrogen production. Int J Hydrogen Energy. 2021;46(31):16622-16630.
Tokarska KB, Gillett, NP. Cumulative carbon emissions budgets consistent with 1.5˚C global warming. Nat Clim Change. 2018;8:296-299.
Zhu X, Imtiaz Q, Donat F, Müller CR, Li F. Chemical looping beyond combustion – a perspective. Energ Environ Sci. 2020;13:772-804.
DOAE. Production and marketing of oil palm in 2018 [Internet]. Bangkok: Department of Agricultural Extension; 2018 [cited 2023 Sep 15]. Available from: https://www.doae.go.th/en/category/press-release/.
Noparat P, Prasertsan P, O-Thong S, Pan X. Sulfite pretreatment to overcome recalcitrance of lignocellulose for enzymatic hydrolysis of oil palm trunk. Energy Procedia. 2017;138:1122-1127.
Noparat P, Prasertsan P, O-Thong S, Pan X. Dilute acid pretreatment at high temperature of oil palm trunk biomass for enzymatic hydrolysis. Energy Procedia. 2015;79:924-929.
Nasir A, Chen HZ, Wang L. Novel single-step pretreatment of steam explosion and choline chloride to de-lignify corn stover for enhancing enzymatic edibility. Process Biochem. 2020;94:273-281.
Fradinhoa P, Oliveira A, Dominguez H, Torres MD, Sousa I, Raymundo A. Improving the nutritional performance of gluten-free pasta with potato peel autohydrolysis extract. Innov Food Sci Emerg Technol. 2020;63:102374.
Wang H, Xie H, Du H, Wang X, Liu W, Duan Y, et al. Highly efficient preparation of functional and thermostable cellulose nanocrystals via H2SO4 intensified acetic acid hydrolysis. Carbohydr Polym. 2020; 239:116233.
Nimmanterdwong P, Chalermsinsuwan B, Piumsomboon P. Emergy evaluation of biofuels production in Thailand from different feedstocks. Ecol Eng. 2015;74:423-437.
Noparat P, Prasertsan P, O-Thong S. Isolation and characterization of high hydrogen-producing strain Clostridium beijerinckii PS-3 from fermented oil palm sap. Int J Hydrogen Energy. 2011;36:14086-14092.
Mohan V, Srikanth S. Regulatory function of divalent cations in controlling the acidogenic biohydrogen production process. RSC Adv. 2012;2:6576-6589.
Box GEP, Behnken DW. Three level design for the study of quantitative variables. Technometrics. 1960;2:455-475.
Lay JJ. Modeling and optimization of anaerobic digested sludge converting starch to hydrogen. Biotechnol Bioeng. 2000;68:269-278.
O-Thong S, Prasertsan P, Karakashev D, Angelidaki I. Thermophilic fermentative hydrogen production by the newly isolated Thermoanaerobacterium thermosaccharolyticum PSU-2. Int J Hydrogen Energy. 2008;33:1204-1214.
Miyazaki K, Irbis C, Takada J, Matsuura A. An ability of isolated strains to efficiently cooperate in ethanolic fermentation of agricultural plant refuse under initially aerobic thermophilic conditions: Oxygen deletion process appended to consolidated bioprocessing (CBP). Bioresour Technol. 2008;99:1768-1775.
Delgenes JP, Moletta R, Navarro JM. Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehae. Enzym Microb Tech. 1996;9:220-225.
Reaño RL. Assessment of environmental impact and energy performance of rice husk utilization in various biohydrogen production pathways. Bioresour Technol. 2020;299:122590.
Li W, Cheng C, Cao G, Ren N. Enhanced biohydrogen production from sugarcane molasses by adding Ginkgo biloba leaves. Bioresour Technol. 2020;298:122523.
Yin Y, Wang J. Optimization of fermentative hydrogen production by Enterococcus faecium INET2 using response surface methodology. Int J Hydrogen Energy. 2019;33(23):6976-6984.
Sun Y, He J, Yang G, Sun G, Sage V. A review of the enhancement of bio-hydrogen generation by chemicals addition. Catalysts. 2019;9:353.
Mishra P, Krishnan S, Rana S, Singh L, Sakinah M, Wahid Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark-photo fermentative approach to biomass. Energy Strat Rev. 2019;24:27-37.
ThaoVi LV, Salakkam A, Reungsang, R. Optimization of key factors affecting bio-hydrogen production from sweet potato starch. Energy Procedia. 2017;138:973-978.
Engliman NS, Abdul PM, Wu SY, Jahim JM. Influence of iron (II) oxide nanoparticle on biohydrogen production in thermophilic mixed fermentation. Int J Hydrogen Energy. 2017;42:27482-27493.
Gou CY, Guo JB, Lian J, Guo YK, Jiang ZS, Yue L, et al. Characteristics and kinetics of biohydrogen production with Ni2+ using hydrogen-producing bacteria. Int J Hydrogen Energy. 2015;40:161-167.
Mahato RK, Kumar D, Rajagopalan G. Biohydrogen production from fruit waste by Clostridium strain BOH3. Renew Energ. 2020;153:1368-1377.
Martinez-Burgosa WJ, Sydneyb EB, de Paula DR, Medeiros ABP, de Carvalho JC, Soccol VT, et al. Biohydrogen production in cassava processing wastewater using microbialconsortia: Process optimization and kinetic analysis of the microbial community. Bioresour Technol. 2020;309:123331.
Xie GJ, Li BF, Wang Q, Ding J, Ren NQ. Ultrasonic waste activated sludge disintegration for recovering multiple nutrients for biofuel production. Water Res. 2016;93:55-64.
Wang J, Yin Y. Clostridium species for fermentative hydrogen production: An overview. Int J Hydrogen Energy. 2021;46(70):34599-34625.

Published:
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
