Sarutpong Marayart
Department of Biology, Faculty of Science, Srinakharinwirot University, Bangkok, Thailand
Hathaichanok Konmun
Department of Biology, Faculty of Science, Srinakharinwirot University, Bangkok, Thailand
Kongkeat Jampasri
Department of Biology, Faculty of Science, Srinakharinwirot University, Bangkok, Thailand
Sukhumaporn Saeng-ngam
Department of Biology, Faculty of Science, Srinakharinwirot University, Bangkok, Thailand
DOI: https://doi.org/10.14456/apst.2025.21
Keywords: DPPH FRAP Medicinal plants Phenolic compound Strawberry “Pharachatan 80”
Abstract
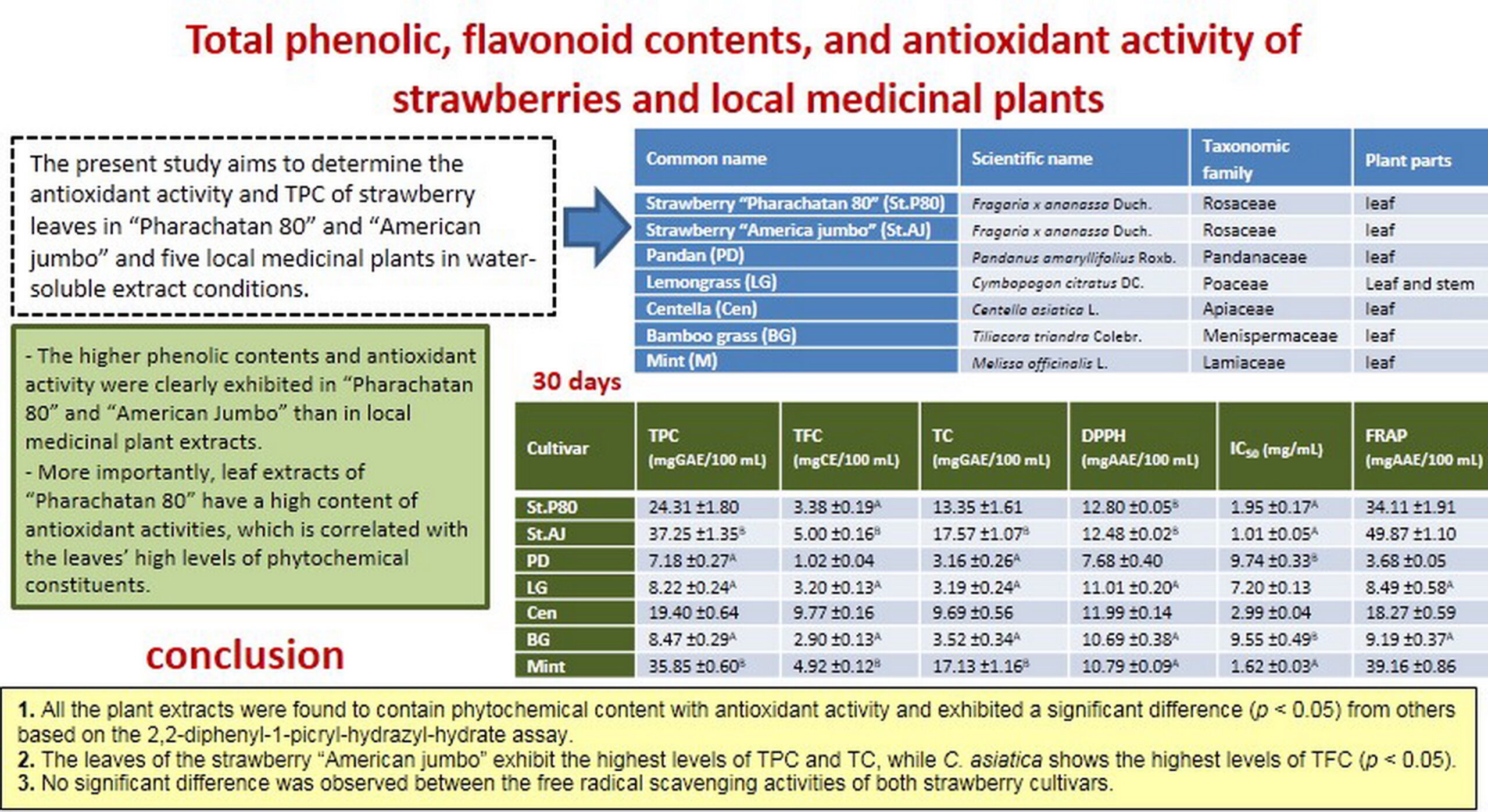
In this study, we assess and compare the total phenolic content (TPC), total flavonoid content (TFC), tannin content (TC), free radical scavenging activity, and ferric reducing antioxidant power in seven local plants. Two strawberry cultivars (Fragaria x ananassa Duch.) with five native medicinal plants, namely, Pandanus amaryllifolius, Cymbopogon citratus, Centella asiatica, Tiliacora triandra, and Melissa officinalis, are investigated. All the plant extracts were found to contain phytochemical content with antioxidant activity. The leaves of the strawberry “American jumbo” exhibit the highest levels of TPC and TC, while C. asiatica shows the highest levels of TFC (p < 0.05). No significant difference was observed between the free radical scavenging activities of both strawberry cultivars. However, these cultivars exhibited a significant difference (p < 0.05) from others based on the 2,2-diphenyl-1-picryl-hydrazyl-hydrate assay. Therefore, this study clearly reveals that water-soluble extracts of strawberry “Pharachatan 80” leaves is the most promising source of potential natural antioxidants among these plant extracts.
How to Cite
Marayart, S. ., Konmun, H., Jampasri, K., & Saeng-ngam, S. (2025). Total phenolic, flavonoid contents, and antioxidant activity of strawberries and local medicinal plants. Asia-Pacific Journal of Science and Technology, 30(02), APST–30. https://doi.org/10.14456/apst.2025.21
References
Lafuente AG, Guillamon E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Infla Res. 2009;58:537-552.
Arts IC, Jacobs DRJ, Gross M, Harnack LJ, Folsom AR. Dietary catechins and cancer incidence among postmenopausal women: the Iowa Women’s Health Study (United States). Cancer Cause Control. 2002;13: 373-382.
Davis W, Lamson MS, Matthew S, Brignall ND: Antioxidants and cancer III: quercetin. Altern Med Rev. 2000;5:196-208.
Taiz L, Zeiger E. Plant Physiology. 5th ed. Massachusetts, USA. Sunderland; Sinauer Associates; 2010. p. 290-296.
Kårlund A, Salminen JP, Koskinen P, Ahern · JR, Karonen M, Tiilikkala K, et al. Polyphenols in strawberry (Fragaria x ananassa) leaves induced by plant activators. J Agric Food Chem. 2014;62:4592-4600.
Rungratanawanich W, Memo M, Uberti D. Redox homeostasis and natural dietary compounds: Focusing on antioxidants of rice (Oryza sativa L.). Nutrients. 2018;10(11):1605.
Chaves VC, Calvete E, Reginatto FH. Quality properties and antioxidant activity of seven strawberry (Fragaria x ananassa duch) cultivars. Sci Hortic. 2017;225:293-298.
Mudnić I, Modun D, Brizić I, Vuković J, Generalić I, Katalinić V, et al. Cardiovascular effects in vitro of aqueous extract of wild strawberry (Fragaria vesca, L.) leaves. Phytomedicine. 2009;16(5):462-469.
Kim MJ, Moon Y, Tou JC, Mou B, Waterland NL. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J Food Compos Anal. 2016;49:19-34.
Sirijan M, Sujipuli K, Pipattanawong N, Chaiprasart P. Generation of strawberry hybrid population to enhance the sweetness and firmness of the fruit. J Agric Sci. 2018;49:362-367.
Chan EWC, Lim YY, Chong KL, Tan JBL, Wong SK. Antioxidant properties of tropical and temperate herbal teas. J Food Compos Anal. 2010;23:185-189.
Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicles. Food Chem. 1999;64:555-559.
Siddhuraju P, Manian S. The antioxidant activity and free radicle-scavenging capacity of dietary phenolic extracts from horse gram (Macrotyloma uniflorum (Lam.) Verdc.) seeds. Food Chem. 2007;105:950-958.
Pham PP, Morales NP, Pitaksuteepong T, Hemstapat W. Antioxidant activity of mulberry stem extract: A potential used as supplement for oxidative stress-related disease. Songklanakarin J Sci Technol. 2017;39:407-414.
Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal Biochem. 1996;239:70-76.
Świętek M, Lu YC, Konefał R, Ferreira LP, Cruz MM, Ma YH, et al. Scavenging of reactive oxygen species by phenolic compound-modified maghemite nanoparticles. Beilstein J Nanotechnol. 2019;10:1073-1088.
Oviedo-Solís CI, Sandoval-Salazar C, Lozoya-Gloria E, Maldonado-Aguilera GA, Aguilar-Zavala H, Beltrán-Campos V, et al. Ultraviolet light-C increases antioxidant capacity of the strawberry (Fragaria x ananassa) in vitro and in high-fat diet-induced obese rats. Food Sci Nutr. 2017;5:1004-1014.
Asemave K. Greener chelators for recovery of metals and other application. Org Chem Int. 2018;6:1-7.
Kicel A, Michel P, Owczarek A, Marchelak A, Żyżelewicz D, Budryn G, et al. Phenolic profile and antioxidant potential of leaves from selected Cotoneaster Medik. Species. Molecules. 2016;21:688.
Pyo YH, Lee TC, Logendra L, Rosen RT. Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts. Food Chem. 2004;85:19-26.
Wong PYY, Kitts DD. Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts. Food Chem. 2006;97:505-515.
Taokaenchan N, Areesrisom P, Thonnalak T, Suthon W, Areesrisoom K. Effects of harvest maturity and light intensity on phenolic compound content and antioxidant activity of Pandanus amaryllifolius Roxb. Leaves. Khon Kaen Agr J. 2017;45:433-438.
Zhang Y, Cai P, Cheng G, Zhang Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat Prod Commun. 2022;17: 1-14.
Muniyandi K, George E, Sathyanarayanan S, George BP, Abrahamse H, Thamburaj S, Thangaraj P. Phenolics, tannins, flavonoids and anthocyanins contents influenced antioxidant and anticancer activities of Rubus fruits from Western Ghats. India. Food Sci Hum Wellness. 2019;8:73-81.
Sarawong C, Schoenlechner R, Sekiguchi K, Berghofer E, Ng PKW. Effect of extrusion cooking on the physicochemical properties, resistant starch, phenolic content and antioxidant capacities of green banana flour. Food Chem. 2014;143:33-39.
Sopittummakhun K, Rattanasinganchan P. Extraction and determination of antioxidant activity in herbal plant. Huachiew Chalermprakiet Sci Technol J.2017;3:86-94.
Ben Ahmed Z, Yousfi M, Viaene J, Dejaegher B, Demeyer K, Mangelings D, et al. Seasonal, gender and regional variations in total phenolic, flavonoid, and condensed tannins contents and in antioxidant properties from Pistacia atlantica ssp. Leaves. Pharm Biol. 2017;55:1185-1194.
Pinheiro DF, Resende JT, Constantino LV, Hata FT, Hata NN, Lustosa SB. Physical, biochemical, and sensory properties of strawberries grown in high-altitude tropical climate. Ciência e Agrotecnologia. 2021;45:1-17.
Li W, Hydanmaka AW, Lowry L, Beta T. Comparison of antioxidant capacity and phenolic compounds of berries, chokecherry and seabuckthorn. Cent Eur J Biol. 2009;4:499-506.
Koca I, Karadeniz B. Antioxidant properties of blackberry and blueberry fruits grown in the Black Sea Region of Turkey. Sci Hortic. 2009;121:447-450.

Published:
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
