Ta T. M. Ngoc
Department of Food Technology, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet Street, District 10, Ho Chi Minh City, Vietnam
Nguyen H. D. Tan
Department of Food Technology, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet Street, District 10, Ho Chi Minh City, Vietnam
Huynh T. N. Ha
Department of Food Technology, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet Street, District 10, Ho Chi Minh City, Vietnam
Nguyen T. M. Nhu
Department of Food Technology, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet Street, District 10, Ho Chi Minh City, Vietnam
Nguyen H. Ngan
Faculty of Food Technology, Nha Trang University, Khanh Hoa Province, Vietnam
DOI: https://doi.org/10.14456/apst.2025.45
Keywords: By-product upgradation Chitin recovery Shrimp head Yarrowia lipolytica Yeast fermentation
Abstract
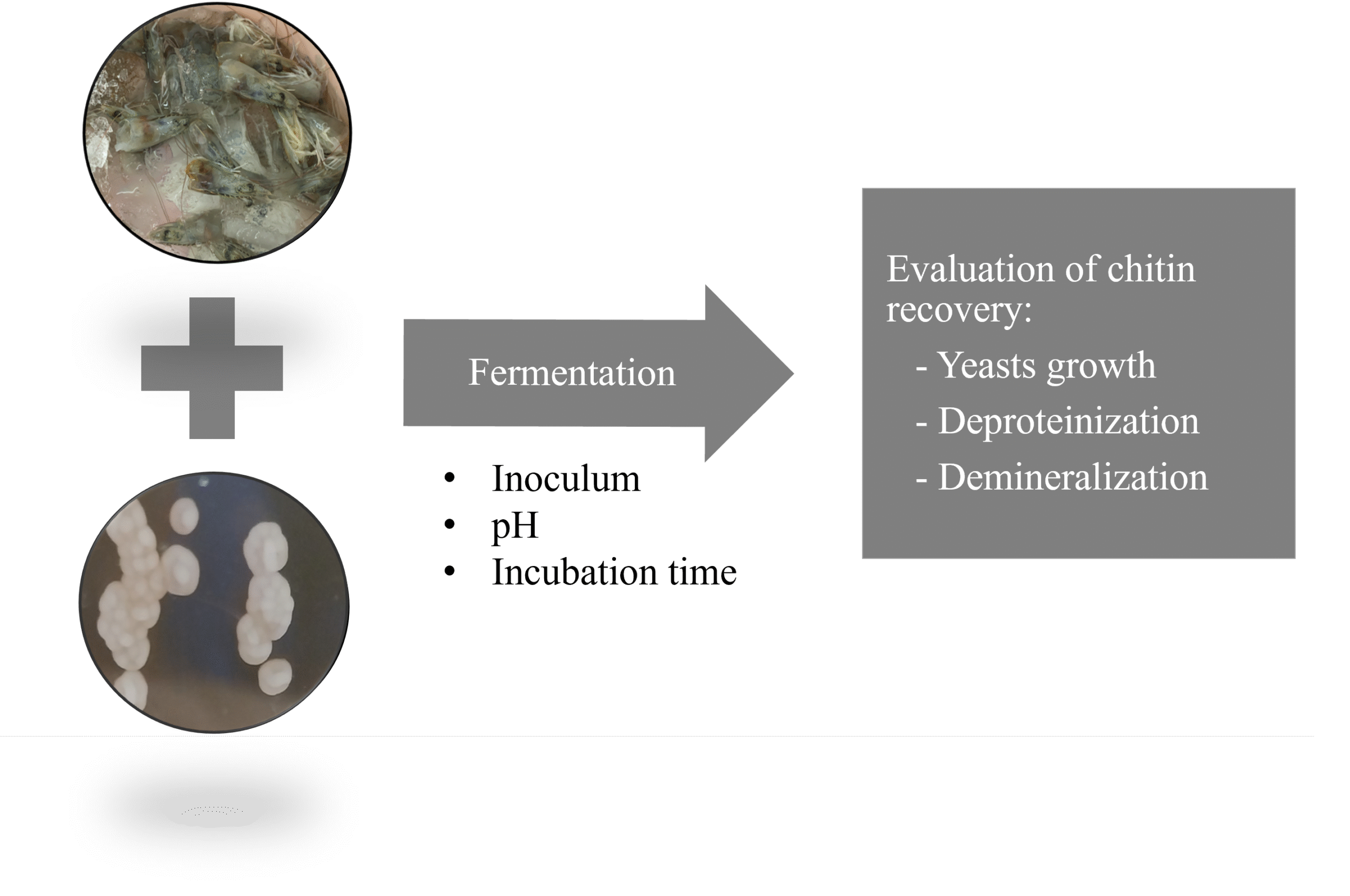
Yarrowia lipolytica is an oleaginous yeast strain proficient in producing extracellular protease and acid, and it has demonstrated the capacity to recover chitin from shrimp by-products. This study examined the submerged fermentation parameters, including incubation time, pH, and inoculum size, utilizing Y. lipolytica to extract chitin from whiteleg shrimp heads. The fermentation efficacy was evaluated through deproteinization and demineralization levels. The results indicated that Y. lipolytica had a notable capacity for synthesizing extracellular protease enzymes, although its potential to acidify the shrimp head environment is minimal. An increment of the deproteinization level with the incubation time, in parallel with the protease activity of the culture, was observed. The suitable pH for fermentation was determined to be 6.0. An inoculum size between 6 and 8 log CFU/mL showed an unclear effect on fermentation efficacy. The deproteinization level reached 91.61±0.54%, while the demineralization level was moderate at 47.17±1.71%.
How to Cite
Ngoc, T. T. M. ., Tan, N. H. D. ., Ha, H. T. N. ., Nhu, N. T. M. ., & Ngan, N. H. . (2025). Effect of culture conditions on chitin recovery from shrimp heads by the oleaginous yeast Yarrowia lipolytica . Asia-Pacific Journal of Science and Technology, 30(03), APST–30. https://doi.org/10.14456/apst.2025.45
References
Sachindra NM, Mahendrakar NS. Process optimization for extraction of carotenoids from shrimp waste with vegetable oils. Bioresour Technol. 2005;96(10):1195-1200.
Mao X, Guo N, Sun J, Xue C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J Clean Prod. 2017;143:814-823.
Zhang D, Wei S, Sun Q, Xia Q, et al. Comparison of the proximate composition and nutritional profile of by-products and edible parts of five species of shrimp. Foods. 2021;10(11):2603.
Ta TMN, Bui HH, Trinh TTN, Nguyen TMN, Nguyen HN. Investigation of chitin recovery from shrimp waste by yeast fermentation. IOP Conf Ser Earth Environ Sci. 2023;1155(1):012012.
Arbia W, Arbia L, Adour L, Amrane A. Chitin extraction from crustacean shells using biological methods – a review. Food Technol Biotechnol. 2013;51(1):12-25.
Khanafari A, Marandi R, Sanatei S. Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods. J Environ Health Sci Eng. 2008;5(1):19-24.
Kaur S, Dhillon GS. Recent trends in biological extraction of chitin from marine shell wastes: a review. Crit Rev Biotechnol. 2015;35(1):44-61.
Rakshit S, Mondal S, Pal K, Jana A, Soren JP, Barman P, et al. Extraction of chitin from Litopenaeus vannamei shell and its subsequent characterization: an approach of waste valorization through microbial bioprocessing. Bioprocess Biosyst Eng. 2021;44(9):1943-1956.
Coelho M, Amaral P, Belo I. Yarrowia lipolytica: an industrial workhorse. In: Méndez-Vilas A, editor. Current research, technology and education topics in applied microbiology and microbial biotechnology. Formatex Res Cent. 2010;930-940.
Nicaud J-M. Yarrowia lipolytica. Yeast. 2012;29(10):409-418.
Morris LS, Evans J, Marchesi JR. A robust plate assay for detection of extracellular microbial protease activity in metagenomic screens and pure cultures. J Microbiol Methods. 2012;91(1):144-146.
Funnekotter B, Mancera RL, Bunn E. A simple but effective combination of pH indicators for plant tissue culture. Plants. 2023;12(4):740.
Kalahroudi RJ, Valizadeh V, Atyabi SM, Keramati M, Cohan RA, Aghai A, et al. Increment in protease activity of Lysobacter enzymogenes strain by ultra violet radiation. Iran J Microbiol. 2020;12(6):601-606.
Denimal E, Marin A, Guyot S, Journaux L, Molin P. Reliable detection and smart deletion of Malassez counting chamber grid in microscopic white light images for microbiological applications. Microsc Microanal. 2015;21(4):886-892.
Zhang Q, Wang L, Liu S, Li Y. Establishment of successive co-fermentation by Bacillus subtilis and Acetobacter pasteurianus for extracting chitin from shrimp shells. Carbohydr Polym. 2021;258:117720.
Suzzi G, Lanorte MT, Galgano F, Andrighetto C, Lombardi A, Lanciotti R, et al. Proteolytic, lipolytic and molecular characterisation of Yarrowia lipolytica isolated from cheese. Int J Food Microbiol. 2001;69(1-2):69-77.
Ozturkoglu-Budak S, Wiebenga A, Bron PA, de Vries RP. Protease and lipase activities of fungal and bacterial strains derived from an artisanal raw ewe’s milk cheese. Int J Food Microbiol. 2016;237:17-27.
Barth G, Gaillardin C. Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol Rev. 1997;19(4):219-237.
Carsanba E, Papanikolaou S, Fickers P, Agirman B, Erten H. Citric acid production by Yarrowia lipolytica. In: Sibirny A, editor. Non-conventional yeasts: from basic research to application: Springer Cham. 2019; 91-117.
Nguyen TMN, Le THT, Pham NT, Tran THH, Le TT, Chau TDA, Ta TMN. Deproteinization and demineralization of shrimp head using yeasts isolated from Nemchua. VNUHCM J Eng Technol. Forthcoming 2025.
Fickers P, Cheng H, Sze Ki Lin C. Sugar alcohols and organic acids synthesis in Yarrowia lipolytica: Where are we? Microorganisms. 2020;8(4):574.
Ciurko D, Neuvéglise C, Szwechłowicz M, Lazar Z, Janek T. Comparative analysis of the alkaline proteolytic enzymes of Yarrowia clade species and their putative applications. Int J Mol Sci. 2023; 24(7):6514.
Nelson G, Young TW. Extracellular acid and alkaline proteases from Candida olea. Microbiology. 1987;133(6):1461-1469.
Glover DJ, McEwen RK, Thomas CR, Young TW. pH-regulated expression of the acid and alkaline extracellular proteases of Yarrowia lipolytica. Microbiology. 1997;143(9):3045-3054.
Ghorbel-Bellaaj O, Younes I, Maâlej H, Hajji S, Nasri M. Chitin extraction from shrimp shell waste using Bacillus bacteria. Int J Biol Macromol. 2012;51(5):1196-1201.

Published:
License
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
